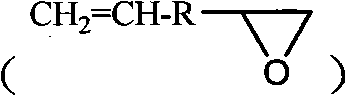Method for preparing organic silicon-modified collagen material
A collagen and silicone technology, applied in chemical instruments and methods, transportation and packaging, dissolution, etc., can solve problems such as poor water resistance, and achieve the effect of reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
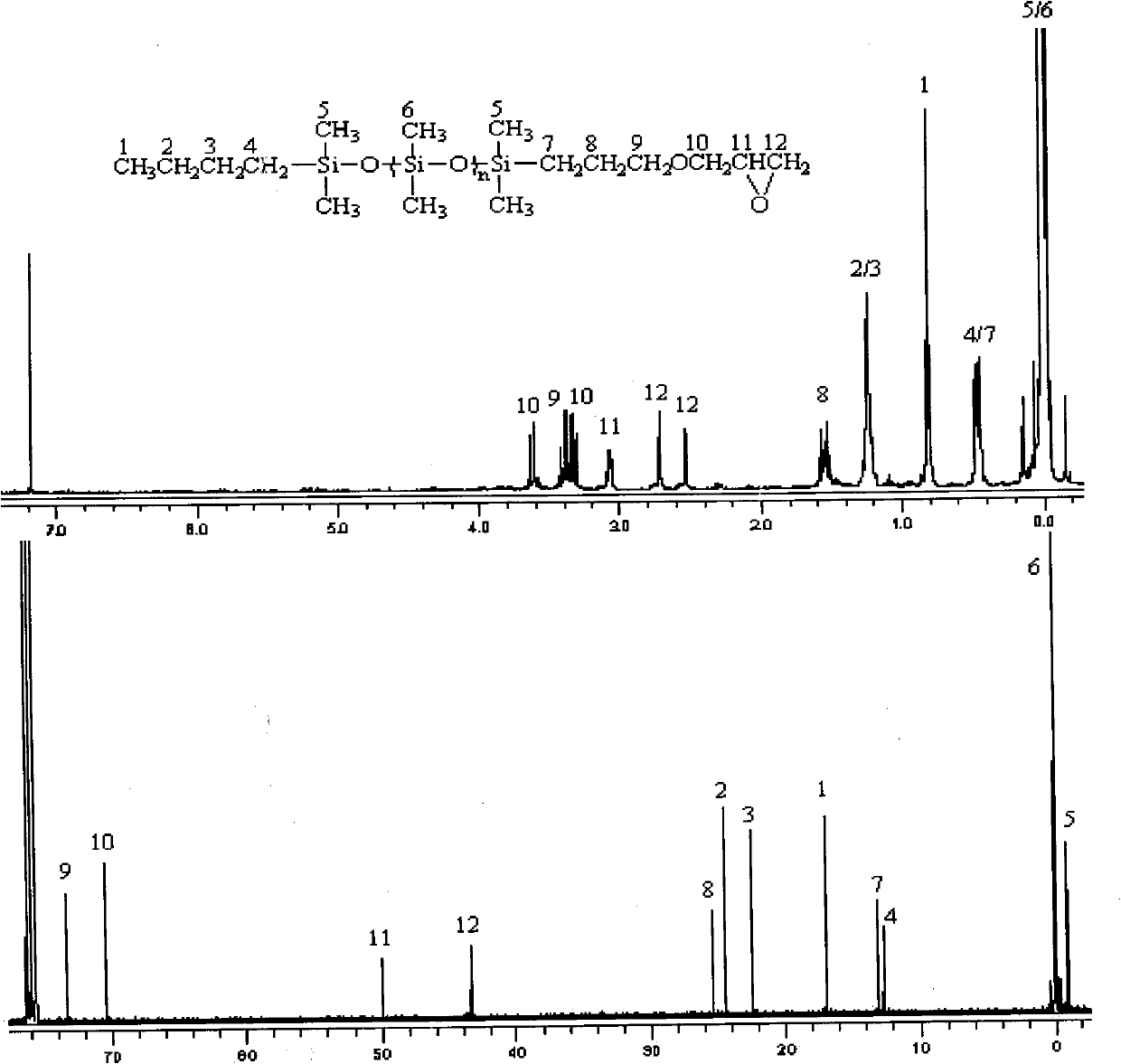
[0030] Anionic ring-opening polymerization (molar ratio hexamethylcyclotrisiloxane: n-butyl lithium: dimethylhydrogen monochlorosilane = 4:1:2)
[0031] In the 250ml reaction flask after anhydrous and anaerobic treatment, add 0.02mol n-butyllithium, 30mL benzene solution containing 17.64g hexamethylcyclotrisiloxane and 40ml tetrahydrofuran successively with a syringe, and stir at 25°C After reacting for 8 hours, 0.04 mol of dimethylhydrogen monochlorosilane was added with a syringe to terminate the reaction. The reaction solution was filtered to remove the generated lithium chloride, and the low boilers were distilled off under reduced pressure to obtain 19.36 g of polysiloxane (Mn=1000) containing a silicon hydrogen group at one end of a colorless transparent liquid with a yield of 97%.
[0032] Hydrosilylation reaction (molar ratio polysiloxane: allyl glycidyl ether = 1:1.2)
[0033] In a 100ml four-neck flask, add compound (I) 1.41g, 10mL toluene and 40μL chloroplatinic ac...
Embodiment 2
[0039] Change the amount of hexamethylcyclotrisiloxane added in the anionic ring-opening polymerization reaction of Example 1 to 28.03 g, 0.02 mol of n-butyllithium, and 0.04 mol of dimethylhydrogen-chlorosilane to terminate the reaction, and other reaction conditions are as in the example As described in 1, 28.50 g (Mn=1500) of polysiloxane containing silicon hydrogen groups at one end was obtained, with a yield of 93.9%. The unsaturated epoxy compound in the hydrosilylation reaction changes from compound (I) to compound (II), and the molar ratio changes from 1: 1.2 to 1: 1. Other conditions are as described in Example 1 to obtain molecular weight and structure Different from the monoepoxy-terminated polysiloxane of Example 1, the yield was 92%. In the preparation process of the organosilicon-modified collagen material in Example 1, the mixed solvent used is changed from water to tetrahydrofuran to water to acetone, the pH is changed to 9.0, and the reaction time is changed t...
Embodiment 3
[0041] In the anionic ring-opening polymerization reaction of Example 1, the amount of hexamethylcyclotrisiloxane added was changed to 19.60 g, the initiator was changed to 0.01 mol of sec-butyl lithium, the accelerator was changed to dimethyl sulfoxide, and 0.02 mol of dimethyl The reaction was terminated by hydrochlorosilane, and the reaction time was changed to 5 h. Other reaction conditions were as described in Example 1, and 19.56 g (Mn=2000) of polysiloxane containing a single-terminal silicon hydrogen group was obtained, with a yield of 94%. The unsaturated epoxy compound in the hydrosilylation reaction is changed from compound (I) to compound (III), the conditions of the hydrosilylation reaction are as described in Example 1, and the molecular weight and structure are different from those of Example 1. Monoepoxy-terminated polysiloxane with a yield of 93%. During the preparation process of the silicone-modified collagen material, the reaction conditions were as describ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com