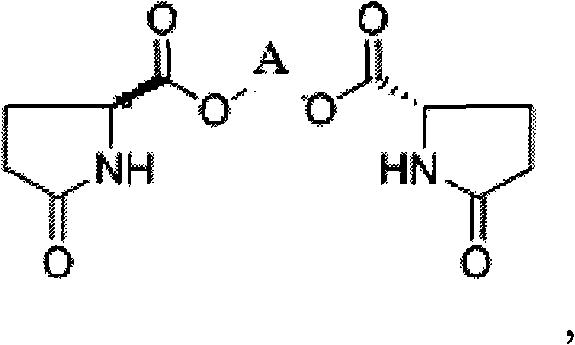
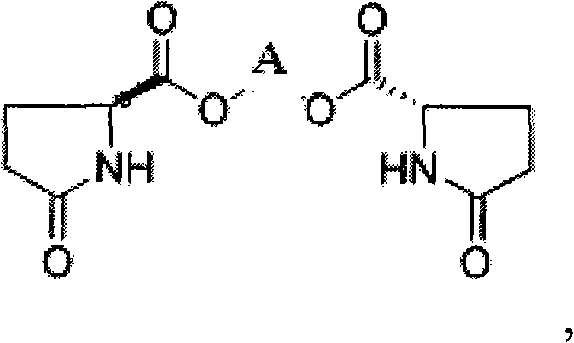
Method for producing L-pyroglutamic acid bivalent alkaline earth metal salt
A technology of pyroglutamic acid and divalent alkali, which is applied in the production technology field of producing divalent alkaline earth metal salt of L-pyroglutamic acid, can solve the problem of high production cost and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] The preparation of embodiment 1L-zinc pyroglutamate
[0016] In a 500L reaction kettle with a stirring and heating reflux device, add 500L of water, raise the internal temperature to 50°C, add 50kg of L-pyroglutamic acid, stir to dissolve it completely, raise the temperature to 80-85°C, and slowly add 15.9kg Zinc oxide, zinc oxide was added for about 2 hours, and stirring was continued for about 4.5 hours. The reaction is over. Cool down to 40-50°C, add 2kg of activated carbon, stir for 2 hours, stop stirring, and filter to remove the activated carbon. Evaporate under reduced pressure until the moisture content is ≤1%. The product L-zinc pyroglutamate is obtained. The yield is 92%, and the product is a white solid.
Embodiment 2
[0017] Embodiment 2: Preparation of L-calcium pyroglutamate
[0018] In a 500L reactor with stirring and heating reflux device, add 500L of water, raise the internal temperature to 50°C, add 50kg of pyroglutamic acid, stir to dissolve it completely, raise the temperature to 80-90°C, and gradually add 14kg of calcium hydroxide , the addition time of calcium hydroxide was about 1 hour, and stirring was continued for about 2 hours. The reaction is over. Cool down to 40-50°C, add 1 kg of activated carbon, stir for 1 hour, stop stirring, and filter to remove the activated carbon. Evaporate under reduced pressure until the water content is ≤0.1%. The product L-calcium pyroglutamate is obtained. The yield is 95%, and the product is a white solid.
Embodiment 3
[0019] Embodiment 3: the preparation of L-magnesium pyroglutamate
[0020] In a 500L reaction kettle with a stirring and heating reflux device, add 500L of water, raise the internal temperature to 50°C, add 100kg of pyroglutamic acid, stir to dissolve it completely, raise the temperature to 70°C, gradually add 16.8kg of magnesium oxide, add Time is about 0.5 hour, stirring is continued for about 4 hours. The reaction is over. Cool down to 40-50°C, add 0.1 g of activated carbon, stir for 1 hour, stop stirring, and filter to remove the activated carbon. Evaporate under reduced pressure at 70°C to foam, then dry under reduced pressure at 0°C, and place in a dry container. The product L-magnesium pyroglutamate was obtained. The yield is 87.5%, and the product is a white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com