Application of lecithin superoxide dismutase composite in preparation of medicines for treating and/or preventing cardiac ischemic injury
A technology of superoxide and cardiac ischemia, applied in drug combinations, cardiovascular system diseases, pharmaceutical formulations, etc., can solve problems such as inability to scavenge oxygen free radicals, difficulty in obtaining ideal results, poor cell affinity, etc., to achieve The effect of reducing reperfusion injury, reducing infarct size, and reducing heart failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
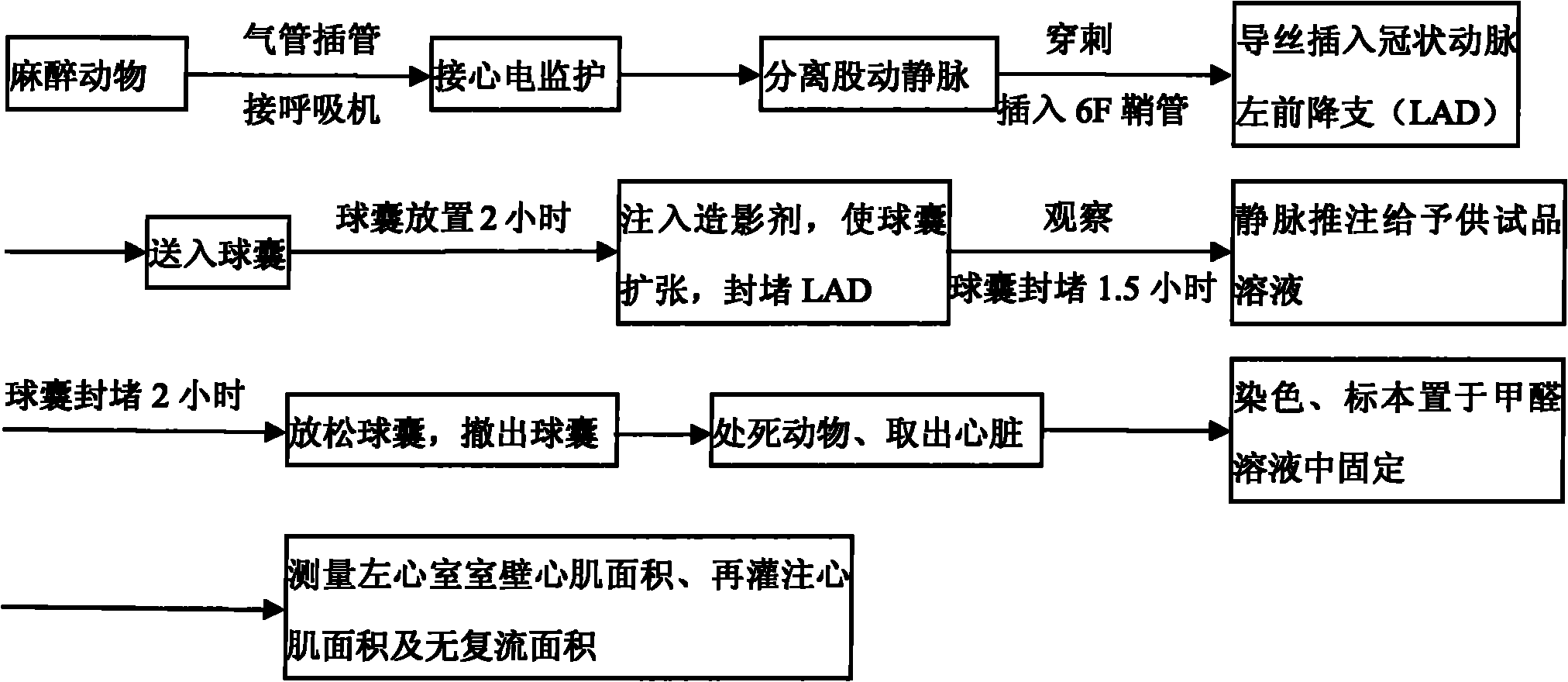
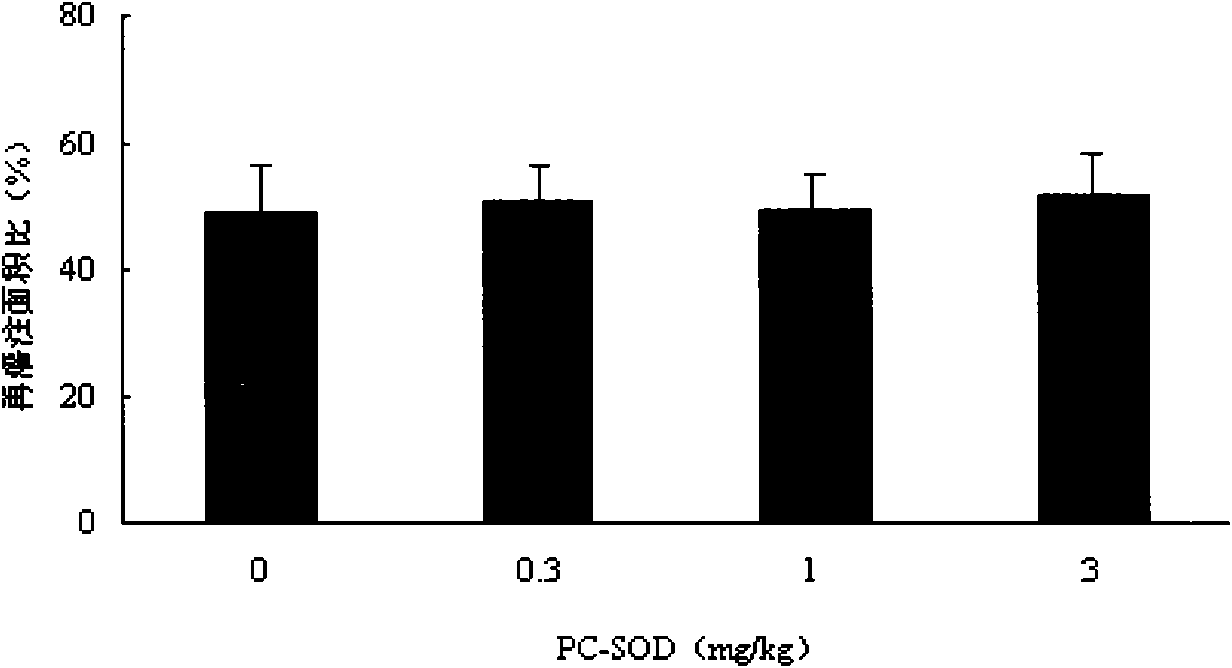
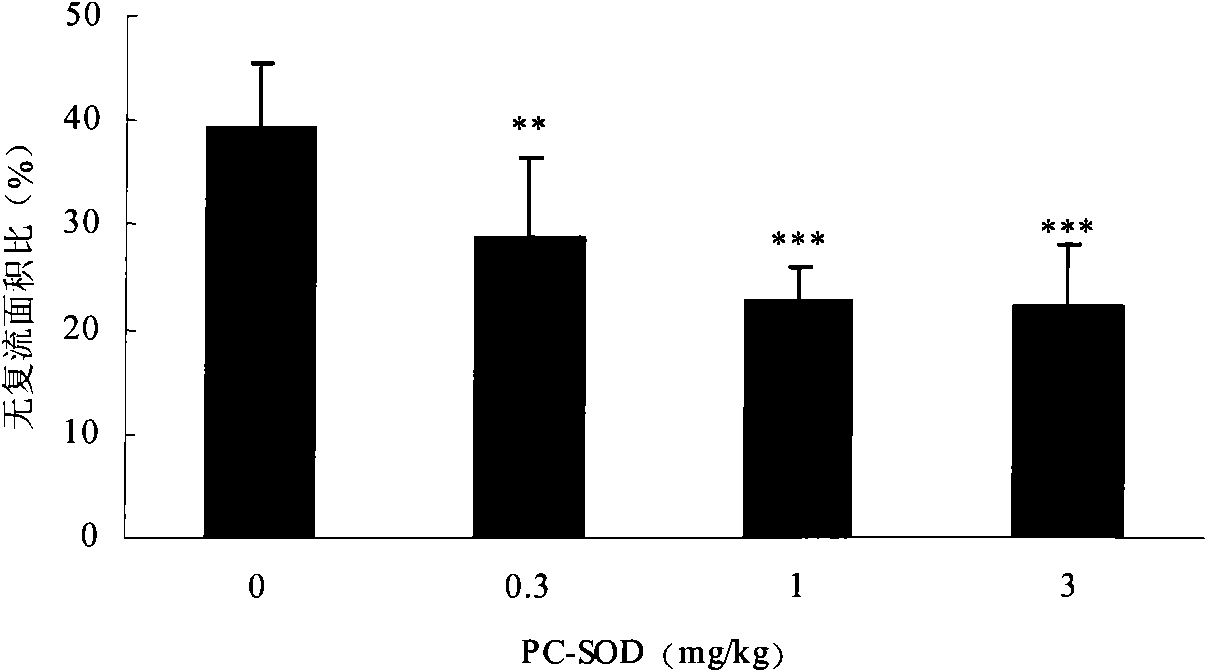
[0028] Example 1 The lecithinated superoxide dismutase composition was administered in different doses, and its pharmacodynamic study was carried out in the treatment of myocardial ischemia-reperfusion in Chinese minipigs
[0029] 1. Experimental materials
[0030] Experimental animals: 35 Chinese miniature pigs, purchased from Beijing Kexing Experimental Animal Breeding Center, male or female, weighing 20-30kg;
[0031] Experimental drug:
[0032] Test product: lecithinated superoxide dismutase composition freeze-dried preparation, its preparation method is as follows:
[0033] 4,500 mL of distilled water for injection was added to a 10 L flask, and 13 g of boric acid, 1.8 g of NaOH, and 68 g of KCl were sequentially added to dissolve. Then, an aqueous SOD solution (50 g, SOD concentration 56.8 mg / mL) was added, and while stirring at 210 rpm, 1000 mL of isopropanol at a temperature of 8° C. was added dropwise at a rate of 16.5 mL / min in the chromatography chamber to prepa...
Embodiment 2
[0075] Example 2 The lecithinated superoxide dismutase composition was administered 30 minutes and 3 minutes before reperfusion, respectively, and its pharmacodynamic study on the treatment of myocardial ischemia and reperfusion in Chinese miniature pigs was carried out
[0076]1. Experimental materials
[0077] Experimental animals: 28 small Chinese animals, purchased from Beijing Kexing Experimental Animal Breeding Center, male or female, weighing 20-30kg;
[0078] Experimental drug:
[0079] Test product: lecithinated superoxide dismutase composition freeze-dried preparation, its preparation method is as follows:
[0080] Add 6000 mL of distilled water for injection into a 20 L flask, and then add 15 g of boric acid, 2.3 g of NaOH and 76 g of KCl in order to dissolve. Then, an aqueous SOD solution (65 g, SOD concentration 60.3 mg / mL) was added, and while stirring at 210 rpm, 1000 mL of isopropanol at a rate of 16.5 mL / min was added dropwise at a temperature of 8° C. in ...
Embodiment 3
[0124] Example 3 Contrasting Pharmacodynamic Activity of PC-SOD Compositions Containing Different Numbers of PC Molecules and PC-SOD Compositions Containing the Same Number of PC Molecules
[0125] In order to verify that the pharmacodynamic activity of PC-SOD compositions containing different numbers of PC molecules is better than that of PC-SOD compositions containing the same number of PC molecules, the present invention measures the pharmacokinetics of blood drug concentration by ELISA method. Kinetic test for comparison, the drug-time curve is as follows Figure 6 shown.
[0126] 1. Experimental materials
[0127] Experimental animals: The experimental animals were 60 SPF grade Sprague-Dawley (SD) rats, purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., half male and half female, weighing 175-205g;
[0128] Experimental drug:
[0129] testing sample:
[0130] Lecithinated superoxide dismutase composition freeze-dried preparation, its pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com