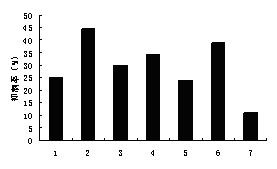Medicinal application of 15-benzyl subunit-1 4-deoxy-11,12-dehydrogenation andrographolide derivative
A technology of andrographolide and derivatives, applied in the field of medicine, to achieve the effect of expanding the range of options and clarifying the anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
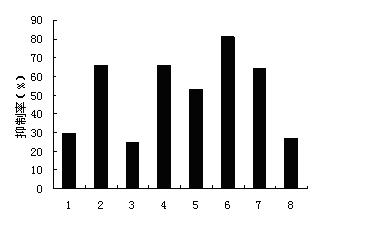
[0018] Example 1 Inhibition of tumor cell clone formation
[0019] 1.1 Materials and methods
[0020] 1) Cell lines: gastric cancer cell line SGC7901, lung cancer cell line A549 and cervical cancer cell line Hela were purchased from Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences.
[0021] 2) Reagents and equipment: RPMI1640 medium, DMEM high glucose medium and trypsin are all GIBICO products. Standard fetal bovine serum (FBS) was purchased from Tianjin Haoyang Biological Products Technology Co., Ltd.; mycoplasma-free fetal bovine serum (FCS) was purchased from Hangzhou Sijiqing Bioengineering Materials Co., Ltd. Andrographolide (1) and andrographolide derivatives (2, 4, 5, 6, 7) synthesized by the present invention were provided by the New Drug Research and Development Center of Zhengzhou University. CO 2 Incubator (German Binder company CB150 type); inverted microscope (Japanese Nikon company TS100-F-PH type). 6-well plates were purchased from Co...
Embodiment 2
[0028] Example 2 Inhibition of Tumor Cell Migration
[0029] 2.1 Materials and methods
[0030] 1) Cell lines: human bladder cancer 5637, human esophageal cancer Ec109, and human colon cancer HT-29 were purchased from Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences
[0031] 2) Reagents and equipment: same as Example 1.
[0032] 3) Test method: Digest conventionally cultured cells with trypsin, spread in a 96-well plate after suspension and dilution, 200 cells per well . After culturing to a confluent monolayer state, replace it with a medium containing 1% serum, and then streak it after 12 hours of synchronous culture, wash it twice with PBS, take pictures under a microscope immediately after adding the drug, and measure the scratch distance. Drug concentrations were 2.50, 5.00, 10.00 , four wells were repeated and a vehicle control was set. After culturing for 24 hours, they were photographed and measured under a microscope, and the migration i...
Embodiment 3
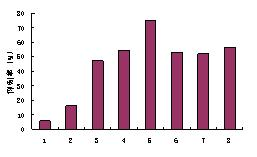
[0035] Example 3 Anti-angiogenic effect
[0036] 3.1 Materials and methods
[0037] 1) Two-dimensional migration of anti-ECV304 (same as Example 2)
[0038] 2) Three-dimensional migration of anti-ECV304
[0039] Serum-free medium was first added to the upper and lower chambers of the Transwell chamber, and placed in a 37°C incubator for 1 hour; human vascular endothelial cells ECV304 (purchased from the Type Culture Collection Center of the Chinese Academy of Sciences) cultured to 80-90% were digested, Resuspend in medium containing 10% fetal bovine serum, adjust cell concentration to 1x10 6 / mL; Take out the medium in the upper and lower chambers of the Transwell chamber, add the above cell solution to the upper chamber, 100 / hole; add 600 to the lower chamber Medium containing 10% serum at 37°C, 5% CO 2 After culturing in the incubator for 2 hours, replace the medium in the upper chamber with serum-free or drug-containing serum-free medium, set 3 replicate wells in ea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com