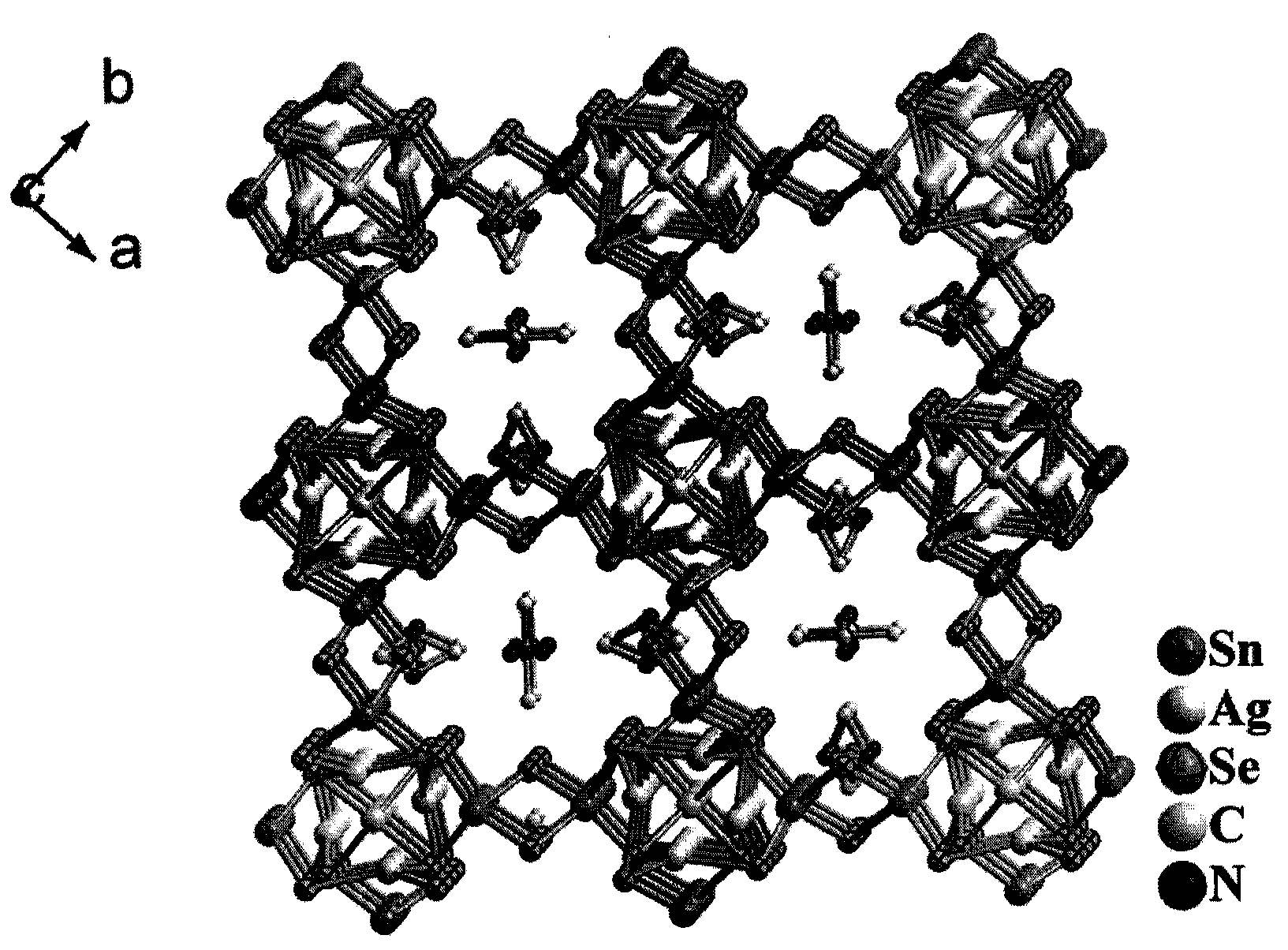
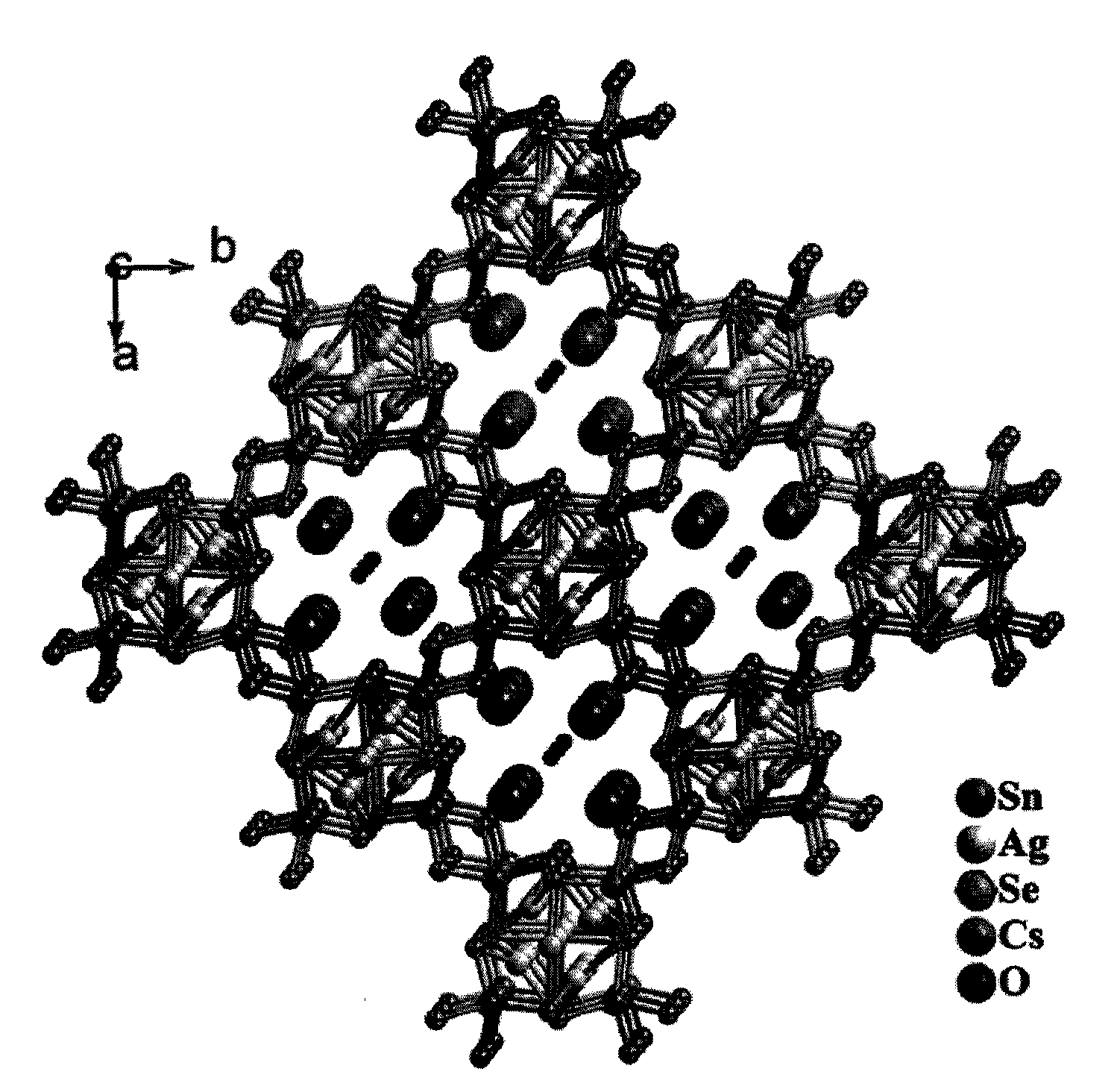
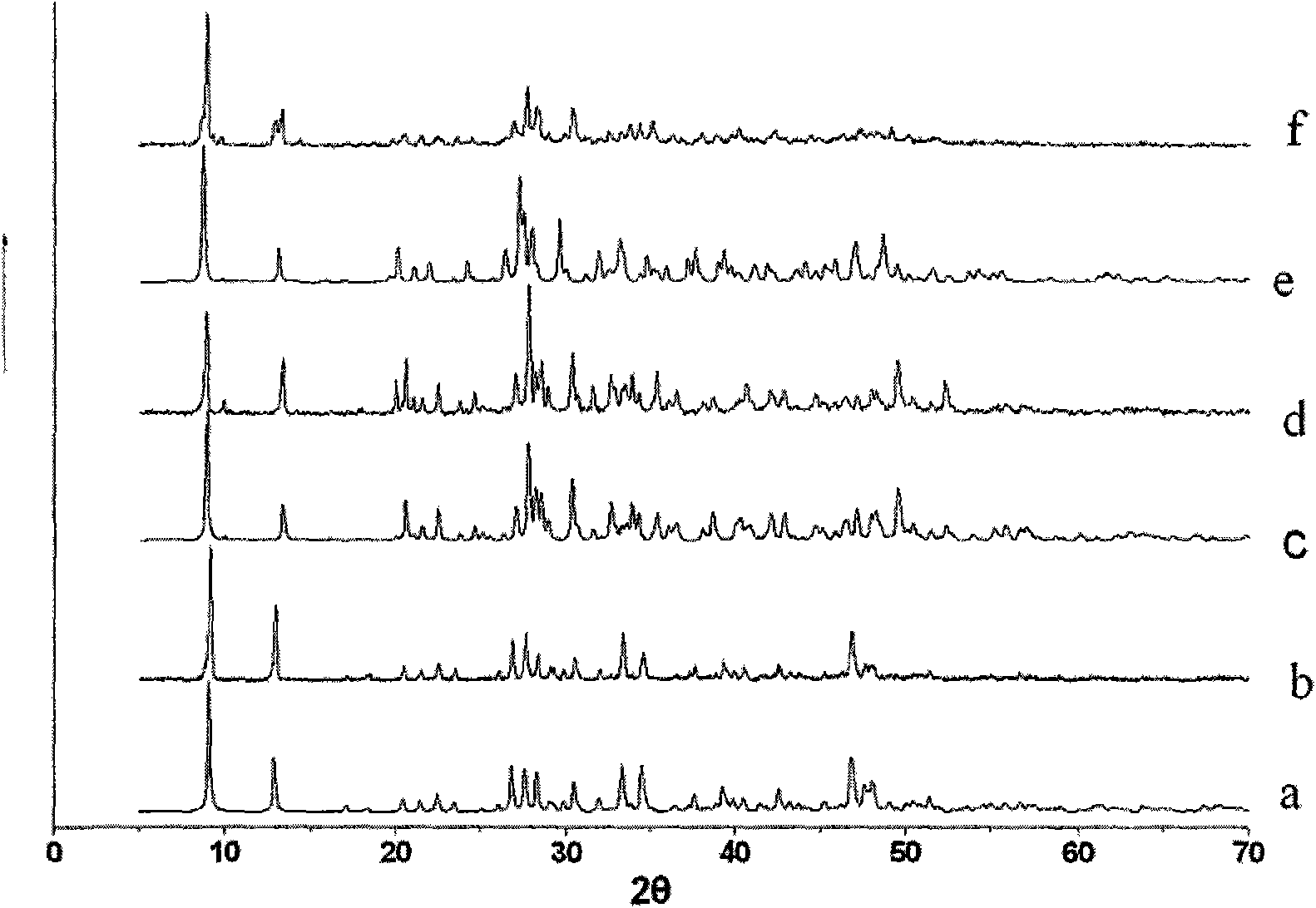
Preparation method and application of novel caesium-removing silver-tin selenide microporous material
A technology of microporous materials, selenides, used in chemical instruments and methods, cation exchange materials, chemical/physical processes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Example 1: Silver-tin selenide [(Me) 2 NH 2 ] 0.75 Ag 1.25 SnSe 3 Preparation
[0013] Add AgCl (1.0mmol, 0.145g), Sn (1.0mmol, 0.114g), Se (3mmol, 0.237g) into 5mL of N,N'-dimethylformamide solvent, fully stir at room temperature and then seal it in the lining In a 28mL stainless steel reactor made of polytetrafluoroethylene, react at a constant temperature of 160°C for 7 days, then naturally cool to room temperature, filter and wash thoroughly with ethanol to obtain dark red block crystals (target product) and a small amount of black powder, crystal product The yield can reach 62%. The same result can be obtained by magnifying the starting reactant by 5 times, indicating that the compound can be prepared in a large amount, which is very important for practical applications.
Embodiment 2
[0014] Example 2: Silver-tin selenide [(Me) 2 NH 2 ] 0.75 A g1.25 SnSe 3 Ion exchange experiment on alkali metals
[0015] A typical ion exchange experiment: the alkali metal chloride ACl (A=Rb, Cs) (2mmol) was dissolved in 20mL of distilled water, and then 100mg[(Me) 2 NH 2 ] 0.75 A g1.25 SnSe 3 For the crystal sample, the mixture is stirred at room temperature for 12 hours, after which the ion exchange product is filtered and thoroughly washed with distilled water, ethanol and ether in sequence to obtain the alkali metal ion exchange product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com