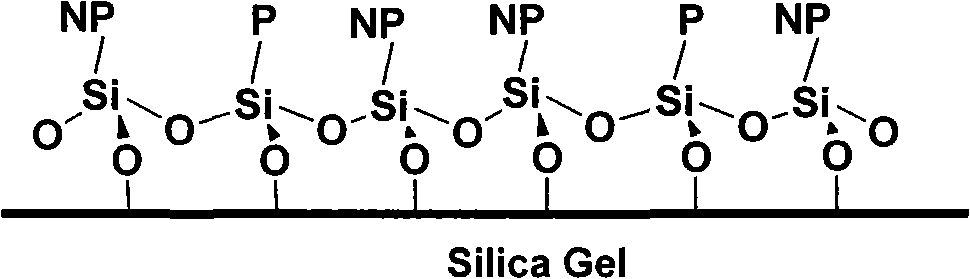
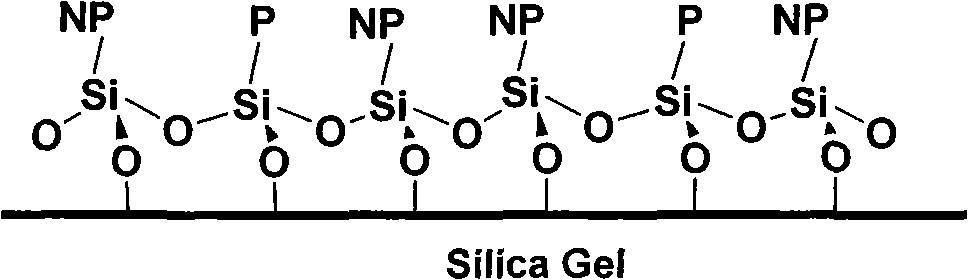
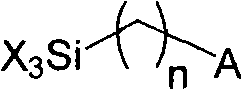
Chromatographic separation material based on copolymerization on silica gel surface and preparation thereof
A technology of copolymerization reaction and chromatographic separation, which is applied in the field of silica gel substrate-bonded "non-polar/polar copolymerization stationary phase" and its preparation, can solve the problems of difficult regulation and fixed spatial position, etc., and achieve large bonding amount and improved Effect of selectivity and wide application of technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Weigh 10g spherical silica gel (particle size is 5μm, pore size is 10nm, specific surface area is 305m 2 / g), placed in a 250mL glass flask, added 150mL of 10% hydrochloric acid solution by volume, heated to reflux for 12 hours, cooled to room temperature, filtered, washed with water until neutral, and dried at 150°C for 24 hours. The dried silica gel was placed in a 150 mL three-neck glass bottle, and nitrogen gas with a relative humidity of 50% was continuously introduced for 48 hours to obtain 10.5 g of hydrated silica gel. Under the condition of passing through dry nitrogen, add 80mL of dry n-hexane to the hydrated silica gel, stir well, then add 18mmol (7.2mL) of octadecyltrichlorosilane and 6mmol (0.9mL) of 3-chloropropyl The trichlorosilane mixture was stirred at room temperature for 24 hours. The reaction system was filtered, washed successively with toluene, dichloromethane, methanol, water, tetrahydrofuran, and methanol, and the product was dried at 80° C. fo...
Embodiment 2
[0026] Its preparation method is: weigh 10g of spherical silica gel (particle size is 3μm, pore size is 12nm, specific surface area is 290m 2 / g), placed in a 250mL glass flask, added 100mL of 38% hydrochloric acid solution, heated to reflux for 2 hours, cooled to room temperature, filtered, washed with water until neutral, and dried at 120°C for 24 hours. The dried silica gel was placed in a 150 mL three-necked glass bottle, and nitrogen gas with a relative humidity of 60% was continuously introduced for 24 hours to obtain 10.3 g of hydrated silica gel. Under the condition of blowing dry nitrogen, add 80mL of dry toluene to the hydrated silica gel, stir well, then add 16mmol (6.4mL) octadecyltrichlorosilane and 8mmol (1.9mL) 3-cyanopropyltrichlorosilane dropwise. Chlorosilane mixture was stirred and reacted at 80°C for 24 hours. The reaction system was filtered, washed successively with toluene, dichloromethane, methanol, water, tetrahydrofuran, and methanol, and the product...
Embodiment 3
[0029] Weigh 10g spherical silica gel (particle size is 5μm, pore size is 10nm, specific surface area is 305m 2 / g), placed in a 250mL glass flask, added 150mL of 10% hydrochloric acid solution, heated to reflux for 12 hours, cooled to room temperature, filtered, washed with water until neutral, and dried at 120°C for 24 hours. The dried silica gel was placed in a 150 mL three-necked glass bottle, and nitrogen gas with a relative humidity of 30% was continuously introduced for 72 hours to obtain 10.6 g of hydrated silica gel. Under the condition of blowing dry nitrogen, add 100mL dry n-pentane to the hydrated silica gel, stir well, then add 16mmol (6.4mL) octadecyltrichlorosilane and 8mmol (1.4mL) 3-aminopropane dropwise Base trimethoxysilane mixture, stirred at room temperature for 24 hours. The reaction system was filtered, washed successively with toluene, dichloromethane, methanol, water, tetrahydrofuran, and methanol, and the product was dried at 80° C. for 12 hours to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com