Thienopyridone derivatives as AMP-activated protein kinase (AMPK) activators
A technology of thiophene and pyridine, applied in the field of thienopyridone derivatives of formula, can solve problems such as energy expenditure and weight loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
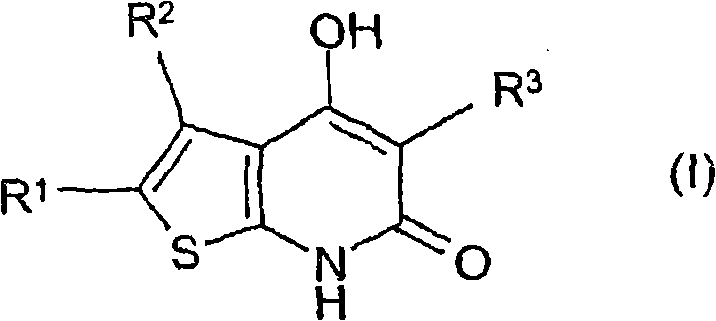
[0394] 4-Hydroxy-3-phenyl-5-(pyridin-3-yl)-6,7-dihydro-thieno[2,3-b]pyridin-6-one
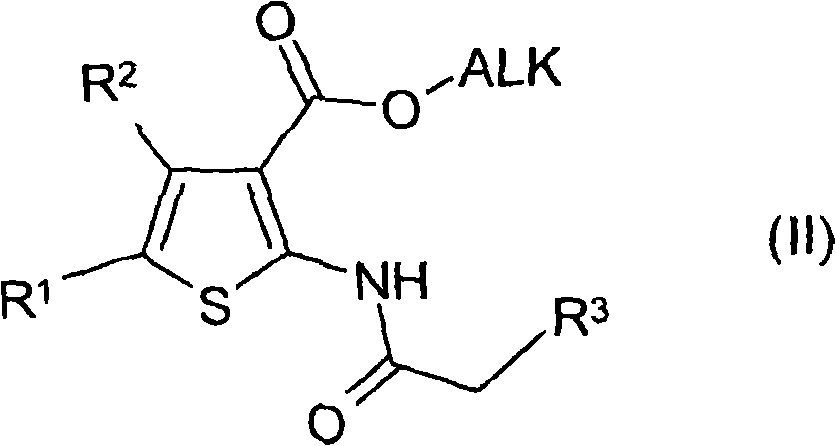
[0395] Step 1: 3-pyridylacetic acid (0.842 g, 4.85 mmol) in acetonitrile (30 mL) was cooled at 0 °C. HBTU (2.169g) and diisopropylethylamine (2.64g) were added. After stirring for 20 minutes, a solution of 2-amino-3-ethoxycarbonyl-4-phenylthiophene (1 g, 4.04 mmol) in acetonitrile was added dropwise. After stirring at room temperature for 15 hours, the solvent was removed under reduced pressure, and the residue was dissolved in dichloromethane. The organic solution was washed with sodium bicarbonate solution, water and then dried over sodium sulfate. The organic solvent was removed under reduced pressure and the crude was purified on silica gel (heptane / ethyl acetate 4 / 6). A yellow oil (407 mg) was recovered.
[0396] 1 HNMR (DMSO-d 6 , 300MHz): 11.45(br.s, 1H), 8.64-8.59(m, 2H), 7.87-7.84(m, 1H), 7.44-7.39(m, 1H), 7.31-7.24(m, 5H), 6.60 (s, 1H), 4.03(q, 2H), 3.88(s, 2H), 0.89(t, 3H)
...
Embodiment 2
[0399] Example 2: 4-Hydroxy-5-(2-hydroxyphenyl)-3-phenyl-2-methyl-6,7-dihydro-thieno[2,3-b]pyridin-6-one
[0400] Step 1: To a solution of propiophenone (30 mL, 0.226 mol) in ethanol (670 mL) was added ethyl cyanoacetate (24 mL) dropwise. After 20 minutes at 60°C, morpholine (68.9 mL) was added and after 5 minutes sulfur (14.5 g) was added. Heated for 72 hours, then the solvent was removed under reduced pressure. The crude was dissolved in dichloromethane, filtered through a pad of silica gel, and the solvent was removed under reduced pressure. The crude was purified over silica gel (heptane / ethyl acetate 9 / 1) and a yellow solid (18.4 g) was recovered;
[0401] 1 HNMR (CDCl 3 , 300MHz): δ(ppm): 7.34-7.27(m, 3H), 7.12-7.15(m, 2H), 3.91(q, 2H), 2.02(s, 3H), 0.78(t, 3H)
[0402] Step 2: to two Add 2-methoxyphenylacetyl chloride (678mg) dropwise to the aforementioned compound (800mg) in alkanes (5mL) solution in alkanes (5 mL). The solution was heated to reflux for 15 ho...
Embodiment 3
[0408] Example 3: 2-Chloro-4-hydroxy-3-(3-methoxyphenyl)-5-phenyl-6,7-dihydro-thieno[2,3-b]pyridin-6-one
[0409] Step 1: Ethyl cyanoacetate was added dropwise to a solution of 3'-methoxyacetophenone (13.7 mL, 0.1 mol) in ethanol (335 mL). After 20 minutes at 60°C, morpholine (30.5 mL) was added and after 5 minutes sulfur (6.4 g) was added. Heated for 72 hours, then filtered through a pad of silica gel, and the solvent was removed under reduced pressure. The crude was purified on silica gel (heptane / ethyl acetate 9 / 1). A yellow solid (3.5 g) was recovered;
[0410] 1 HNMR (CDCl 3 , 300MHz): δ(ppm): 7.26-7.20(m, 1H), 6.88-6.80(m, 3H), 6.07(br.s, 1H), 4.04(q, 2H), 3.81(s, 3H), 0.95(t,3H).
[0411] Step 2: To the aforementioned compound (1.5g, 5.40mmol), two Phenylacetyl chloride (858 μL) was added dropwise to a solution of alkanes (9.3 mL) and pyridine (523 μL). The reaction mixture was heated to 105 °C for 1 hour, then the solvent was evaporated. The crude was dissolv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com