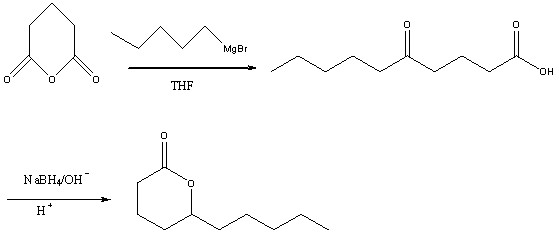
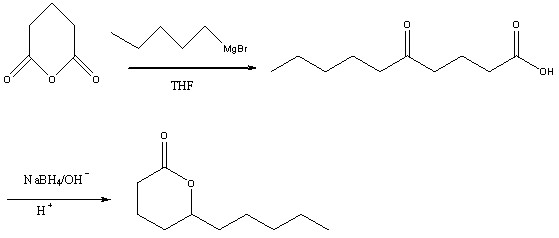
Method for preparing high-purity delta decalactone
A kind of decanolide and high-purity technology, which is applied in the field of preparation of high-purity butyl-decalactone, can solve problems such as difficult industrialized production, complicated reaction route, expensive raw materials, etc., and achieves low price, easy-to-obtain reaction raw materials, and high raw materials. low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Preparation of bromopentane Grignard reagent
[0026] Under the protection of nitrogen, add 200ml of tetrahydrofuran, 10g of magnesium flakes and a small amount of iodine into a 500ml four-necked flask equipped with electromagnetic stirring, a thermometer and a reflux condenser with a drying tube, raise the temperature to 50°C, and add dropwise 60g of tetrahydrofuran dissolved in 40ml of tetrahydrofuran Pentyl bromide, control the temperature not to exceed 70°C, after the dropwise addition, continue to stir for 1 hour to obtain the Grignard reagent of pentyl bromide;
[0027] (2), Preparation of 5-oxodecanoic acid intermediate
[0028] Under the protection of nitrogen, add 300ml of tetrahydrofuran, 36g of glutaric anhydride and 2.8g of cuprous iodide into a 1L four-neck flask, cool to -20°C, add the above-mentioned Grignard reagent of bromopentane dropwise, and the dropping time is About 1h. After the dropwise addition, continue to stir for 2 hours, add 1mol / L hyd...
Embodiment 2
[0032] (1), preparation of chloropentane Grignard reagent
[0033] Under the protection of nitrogen, add 200ml of tetrahydrofuran, 10g of magnesium flakes and a small amount of iodine into a 500ml four-necked flask equipped with electromagnetic stirring, a thermometer and a reflux condenser with a drying tube, raise the temperature to 50°C, and add dropwise 42.7 g pentane chloride, control the temperature not to exceed 70°C, after the dropwise addition, continue to stir for 1 hour to obtain the Grignard reagent of pentane chloride;
[0034] (2), Preparation of 5-oxodecanoic acid intermediate
[0035] Under the protection of nitrogen, add 300ml of tetrahydrofuran, 36g of glutaric anhydride and 2.8g of cuprous iodide into a 1L four-neck flask, cool to -20°C, add the above-mentioned Grignard reagent of chloropentane dropwise, and the dropping time is About 1h, after the dropwise addition, continue to stir for 2h, add 1mol / L hydrochloric acid solution to hydrolyze to pH=2, let st...
Embodiment 3
[0039] (1) Preparation of bromopentane Grignard reagent
[0040] With embodiment 1;
[0041] (2), Preparation of 5-oxodecanoic acid
[0042] Under the protection of nitrogen, add 300ml of tetrahydrofuran, 36g of glutaric anhydride and 4g of copper bromide into a 1L four-neck flask, cool to -20°C, add the above-mentioned Grignard reagent of bromopentane dropwise, and the dropping time is about 1h , after the dropwise addition, continue to stir for 2 hours, add 1mol / L hydrochloric acid solution to hydrolyze to pH = 2, let stand to separate the water layer, extract the water layer twice with methyl tert-butyl ether, combine the organic phases, and saturated chlorine Wash with sodium chloride solution until neutral, dry over anhydrous magnesium sulfate, and remove the solvent under reduced pressure to obtain 45.5 g of crude product 5-oxodecanoic acid;
[0043] (3), the preparation of butyl decalactone
[0044] Add 215g of 5% sodium hydroxide solution and the above-mentioned cru...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com