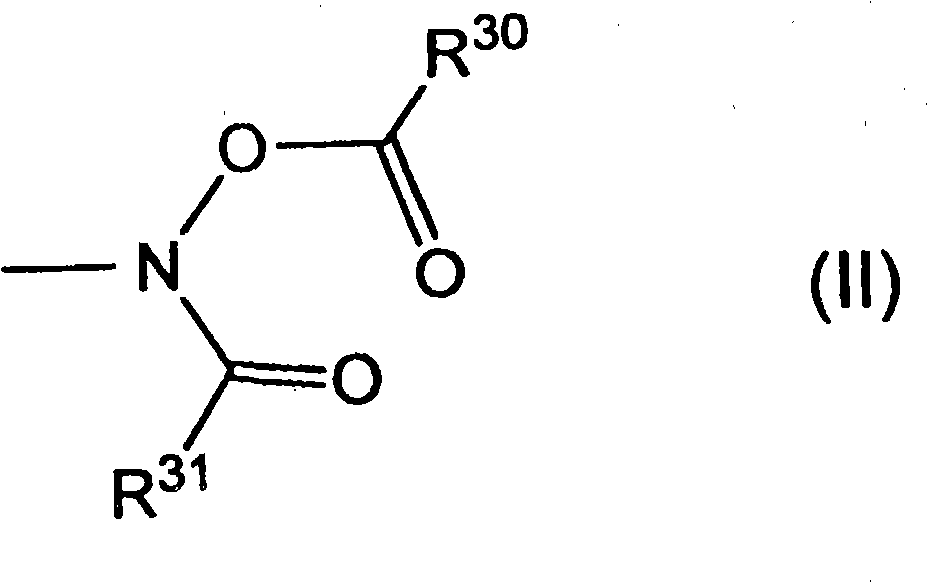
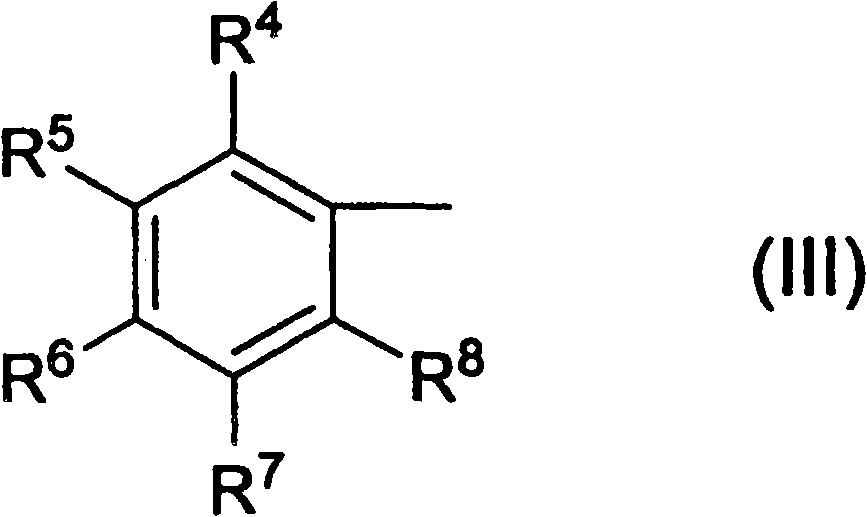
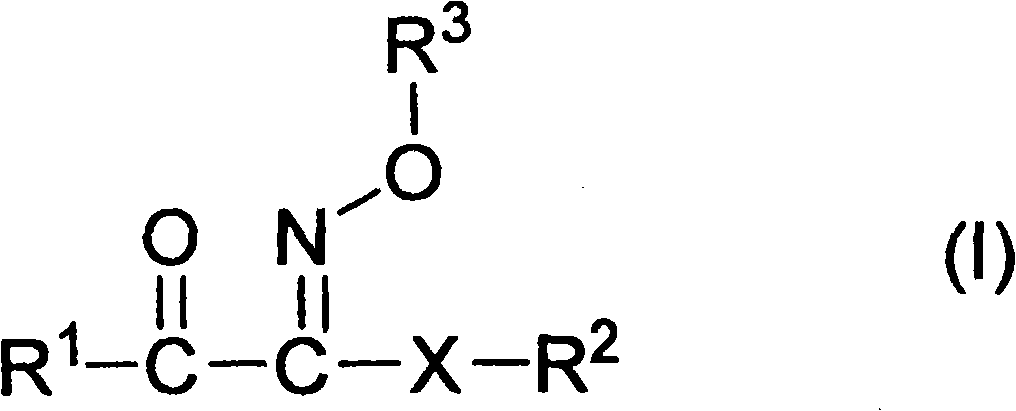Ketoxime ester compound and use thereof
A technology of ester compounds and compounds, which is applied in the field of ketoxime ester compounds and photopolymerizable compositions, can solve the problems of low sensitivity and poor productivity, and achieve the effect of high transmittance, high sensitivity and transmittance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0513] [Example 1] (manufacture of ketoxime ester compound I-1)
[0514]
[0515] Ethylcarbazole (5g, 25.61mmol) and o-naphthoyl chloride (5.13g, 26.89mmol) were dissolved in 30ml of dichloromethane, stirred after cooling to 2°C in an ice-water bath, and aluminum chloride (3.41 g, 25.61 mmol). Stirring was further performed at room temperature for 3 hours. The reaction solution was poured into 200 ml of ice water, 200 ml of dichloromethane was added, and the organic layer was separated. The recovered organic layer was dried over magnesium sulfate and evaporated to give a white solid (10 g). The reaction formula is shown below.
[0516] [chemical formula 25]
[0517]
[0518]
[0519] Dissolve monoketone (7.05g, 22.34mmol) and 3-methylthiopropionyl chloride (3.77g, 21.06mmol) in 100ml of dichloromethane, cool to 2°C in an ice-water bath, stir, and add chloride Aluminum (6.30 g, 40.21 mmol). Stirring was further performed at room temperature for 3.5 h...
Embodiment 2
[0536] [Example 2] (manufacture of ketoxime ester compound 1-2)
[0537]
[0538] According to the same method as in Example 1, obtain 12.4 g of white solid. The reaction formula is shown below.
[0539] [chemical formula 30]
[0540]
[0541]
[0542] According to the same method as in Example 1, 2.0 g of diketone (3.82 g, 8.61 mmol), isoamyl nitrite (1.36 g, 11.62 mmol), and trimethylsilyl chloride (3.12 g, 27.72 mmol) were obtained to obtain 2.0 g of diketone yellow crystals. The reaction formula is shown below.
[0543] [chemical formula 31]
[0544]
[0545]
[0546] According to the same method as in Example 1, 1.6g of the following ketoxime ester compounds were obtained from ketoxime (5.0g, 10.32mmol), acetyl chloride (1.62g, 20.64mmol), triethylamine (2.09g, 20.64mmol) White crystals of I-2.
[0547] [chemical formula 32]
[0548]
[0549] The NMR chemical shift values of this compound are shown below.
[0550] ...
Embodiment 3
[0555] [Example 3] (manufacture of ketoxime ester compound I-8)
[0556] Except that the glutaric acid monoethyl chloride used in the manufacture of the diketone body in the above-mentioned Example 2 was changed to glutaric acid monomethyl chloride, the following ketoxime ester compound I was produced according to the same method as in Example 2- 8. The NMR chemical shift values of the obtained compound are shown below.
[0557] 1 H-NMRδ[ppm]
[0558] 1.49(t, 3H), 2.29(s, 3H), 2.36(s, 3H), 2.73(t, 2H), 3.14(t, 2H), 3.60(s, 3H), 4.43(q, 2H), 7.3 ~7.5(m, 6H), 8.06(dd, 1H), 8.29(dd, 1H), 8.59(d, 1H), 8.83(dd, 1H)
[0559] [chemical formula 34]
[0560]
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| epoxy equivalent | aaaaa | aaaaa |
| softening point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com