Freeze-dried liposome composition of water-soluble medicament and preparation method thereof
A technology for lyophilizing liposomes and water-soluble drugs, applied in the field of medicine, can solve problems such as leakage of water-soluble drugs, inability to store stably for a long time, aggregation and fusion encapsulation rate between particles, etc., so as to avoid drug leakage and improve stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Raw and auxiliary drugs: water-soluble drug is doxorubicin hydrochloride 0.2g, phospholipid is HSPC 0.9g, polyethylene glycol derivatized phospholipid is DSPE-mPEG20000.4g, cholesterol is 0.25g, sugar lyoprotectant is sucrose 14.0 g. Cyclodextrin or cyclodextrin derivative lyoprotectant is 1.25 g of hydroxypropyl-β-cyclodextrin.
[0042] The preparation method is as follows:
[0043] Phospholipids, polyethylene glycol derivatized phospholipids, and cholesterol are dissolved in 500ml of organic solvent 99.5% ethanol to obtain transparent liquid A; transparent liquid A is put into a 60°C water bath, and 250ml of the first buffer ammonium sulfate solution (250mM ) was added to the transparent liquid A while stirring, and the obtained product was extruded with a high-pressure homogenizer to obtain a blank liposome D with an average particle diameter of about 90 nm; a second buffer was added to the blank liposome D obtained. Liquid histidine (10mM) 5000ml replaces the first...
Embodiment 2
[0046] Raw and auxiliary drugs: daunorubicin hydrochloride 0.2g, HSPC1.2g, DSPE-mPEG20000.6g, cholesterol 1.0g, sucrose 13.0g, hydroxypropyl-β-cyclodextrin 2.5g Preparation method: same as Example 1, wherein , the water bath temperature was 76°C, and the encapsulation temperature was 105°C.
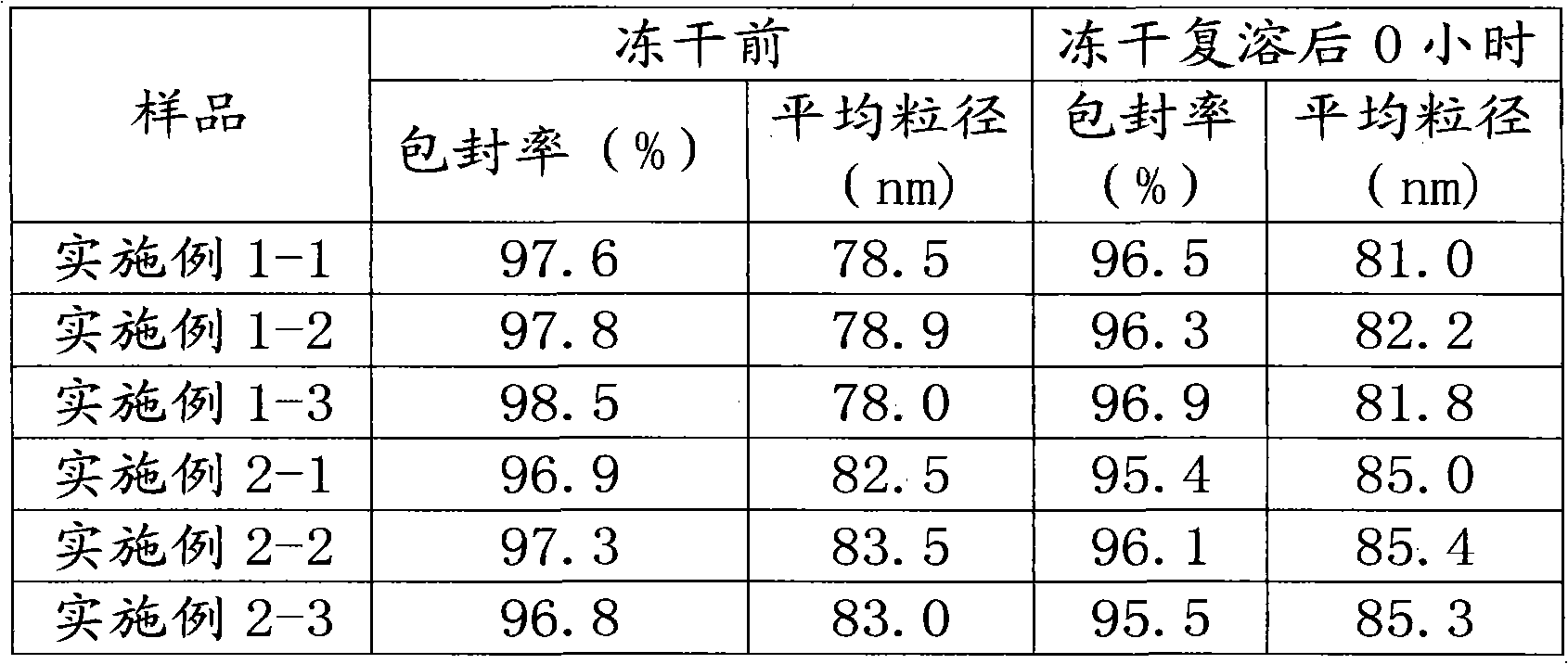
[0047] Three batches of products were prepared, and the obtained products were Examples 2-1, 2-2, and 2-3, respectively.
Embodiment 3
[0048] Example 3 Primary and auxiliary drugs: 0.2g of doxorubicin hydrochloride, 0.32g of HSPC, 0.18g of DSPE-mPEG20000, 0.2g of cholesterol, 13.9g of sucrose, and 0.78g of hydroxypropyl-α-cyclodextrin.
[0049] Preparation method: Same as Example 1, the organic solvent is replaced by 99.5% methanol (1000ml), the second buffer is replaced by citrate (10mM) 250ml; other components remain unchanged; while the water bath temperature is 65 ° C, the encapsulation temperature is 80 ℃.
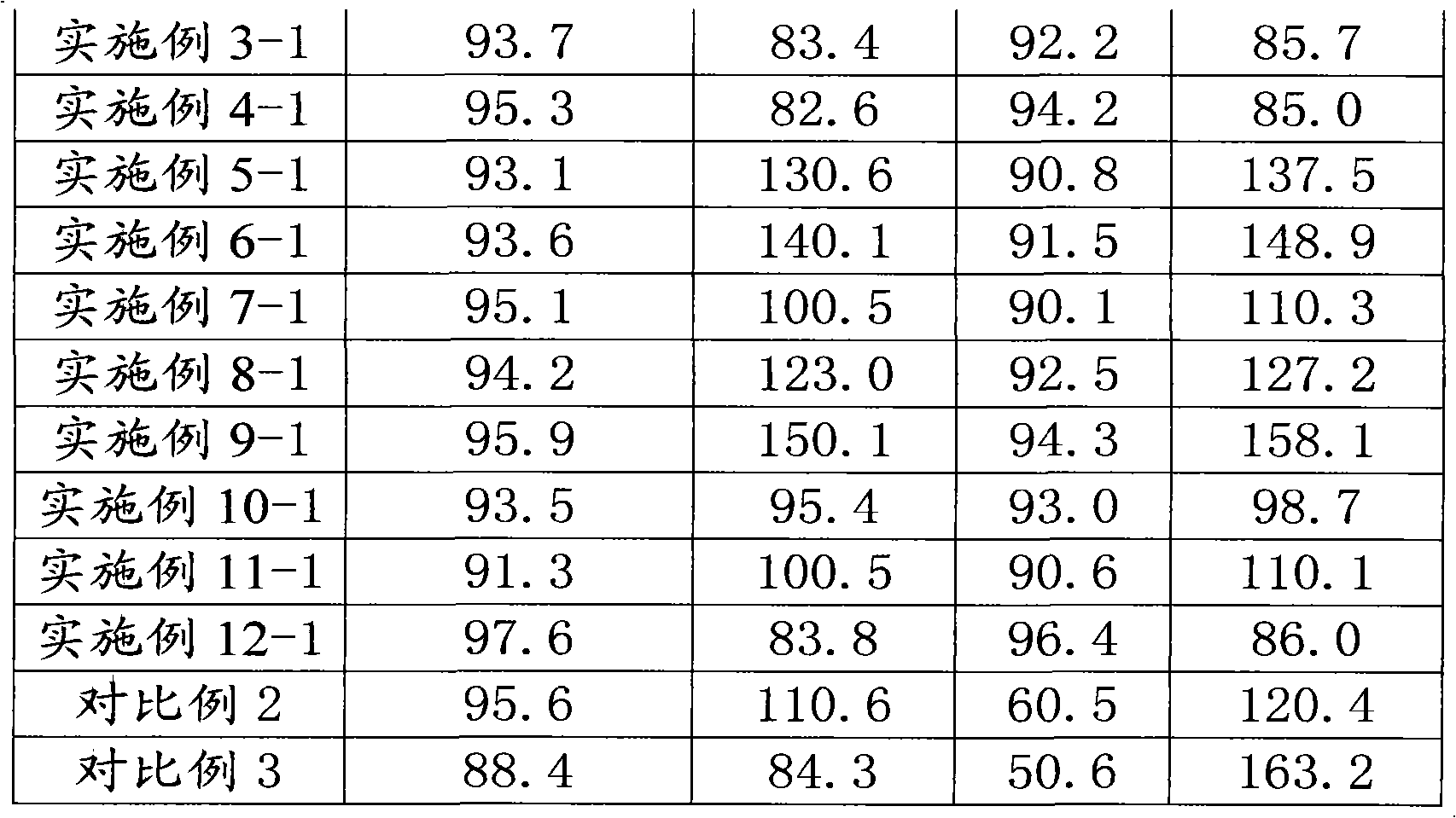
[0050] Three batches of products were prepared, and the obtained products were respectively Example 3-1, Example 3-2, and Example 3-3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com