Method for separating and purifying schwann cells
A technology of Schwann cells and cells, which is applied in the field of cell biology, can solve the problems of cumbersome steps, expensive reagents, and difficulty in peeling off the outer membrane, and achieve obvious effects, simple steps, and economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
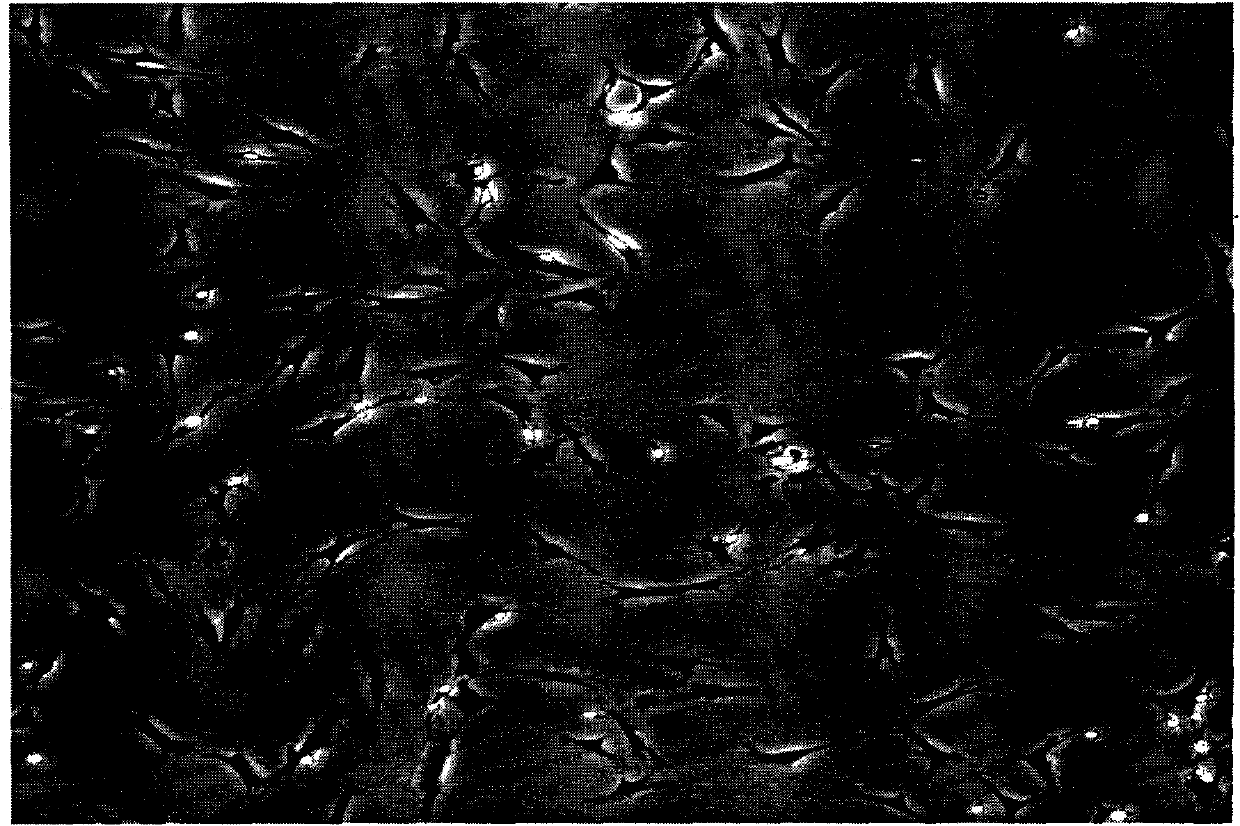
[0037] Example 1 Separation and Purification of Schwann Cells
[0038] Experimental materials: 15ml centrifuge tube, SD mouse, 50ml culture bottle, compound collagenase, dispaseII rabbit anti-mouse polyclonal S100 antibody (primary antibody A), rabbit anti-mouse P75 antibody (primary antibody), goat anti-rabbit PE-labeled antibody (secondary antibody) Anti) etc. Unless otherwise specified, the reagents of the present invention were all purchased from Sigma Company.
[0039] Newborn 6-day-old SD mice were killed by dislocation, put into 75% alcohol, and soaked for 5-10 minutes. Under sterile conditions, the bilateral sciatic nerves with a length of about 1 cm were harvested.
[0040] (1) Under aseptic conditions, the nerve segments of humans or animals are taken, and the epineurium is stripped and removed under a microscope.
[0041] (2) Digest the nerve bundles with a DMEM solution of 0.1-0.2% complex collagenase NB4 and 0.05-0.1% dispase mixed enzyme in a mass volume ratio...
Embodiment 2
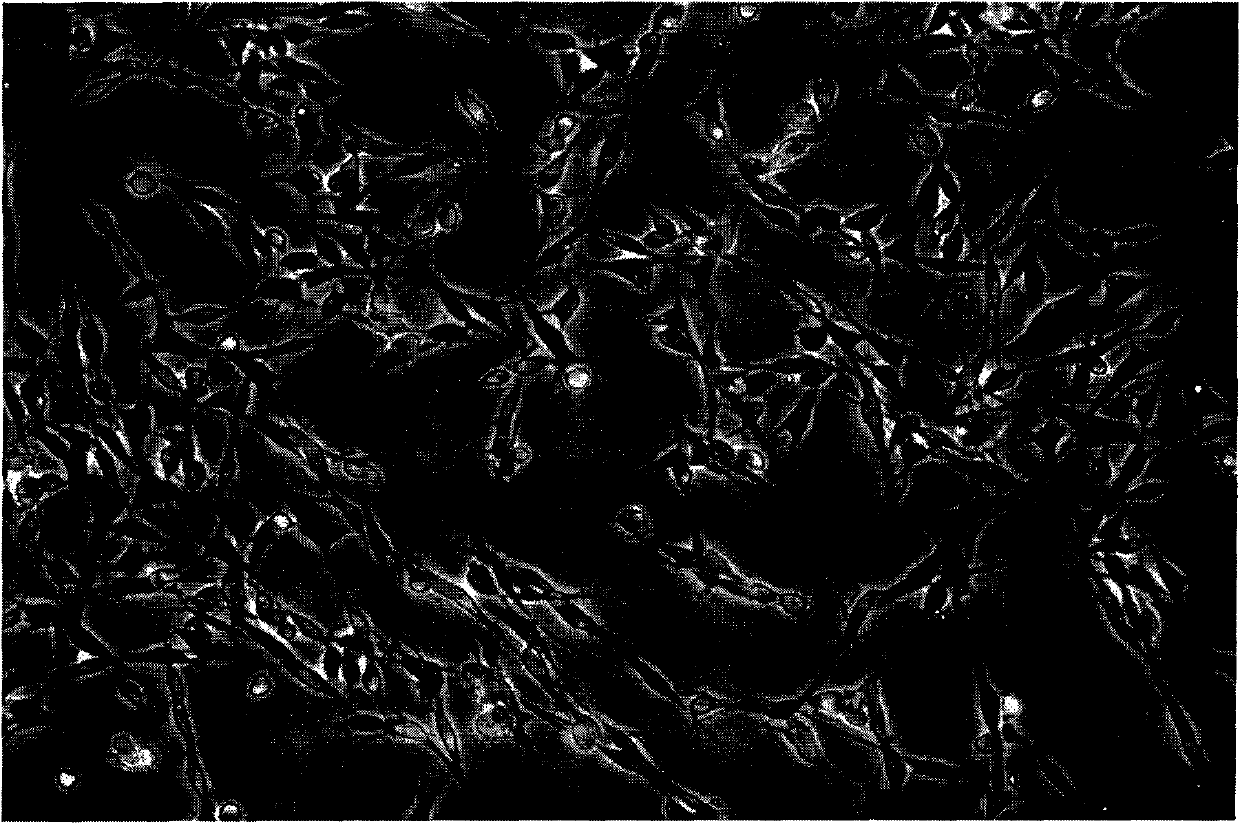
[0051] Embodiment 2 Two kinds of digestive enzyme system digestion results comparison
[0052] experimental method:
[0053] 1) Take primary cells
[0054] 1 Six newborn 6-day-old C57BL6 mice were killed by dislocation, put in 75% alcohol and soaked for 5-10 minutes
[0055] 2 Under sterile conditions, take bilateral sciatic nerves with a length of about 1 cm
[0056] 3. Put 12 segments of nerves into 15ml centrifuge tube A containing 3ml 2% compound collagenase NB4 and 15ml centrifuge tube B containing 2ml 2% compound collagenase NB4 and 1ml 0.1% dispaseII respectively.
[0057] 4 Put it in a 37°C incubator containing 5% CO2, shake it every five minutes, and digest for about 1.5 hours.
[0058] 5 Centrifuge tube A and tube B at 600g for 5 minutes, collect cells, count and plant them into culture flasks, 0.5min per bottle.
[0059] 2) Purification of Schwann cells
[0060] After the primary cells were cultured for 48 hours, the cells were close to confluence: then the obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com