Dual long-chain s-triazine amphoteric surfactants and synthesis method thereof
A technology of surfactant and s-triazine, which is applied in the field of surfactant synthesis chemistry, can solve the problems of great harm and achieve the effect of simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Synthesis of intermediate 2-dodecylamino-4,6-dichloro-1,3,5-s-triazine
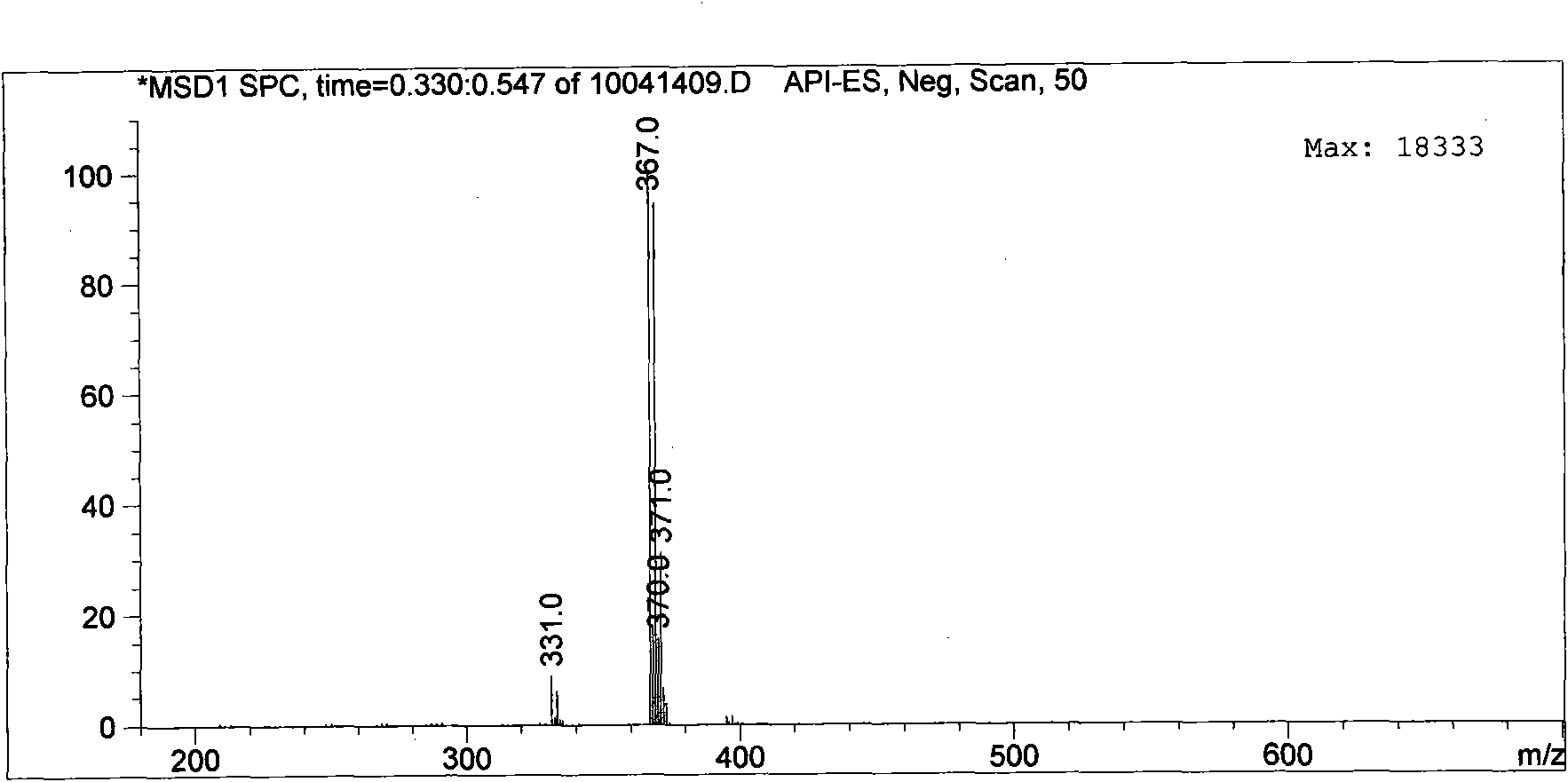
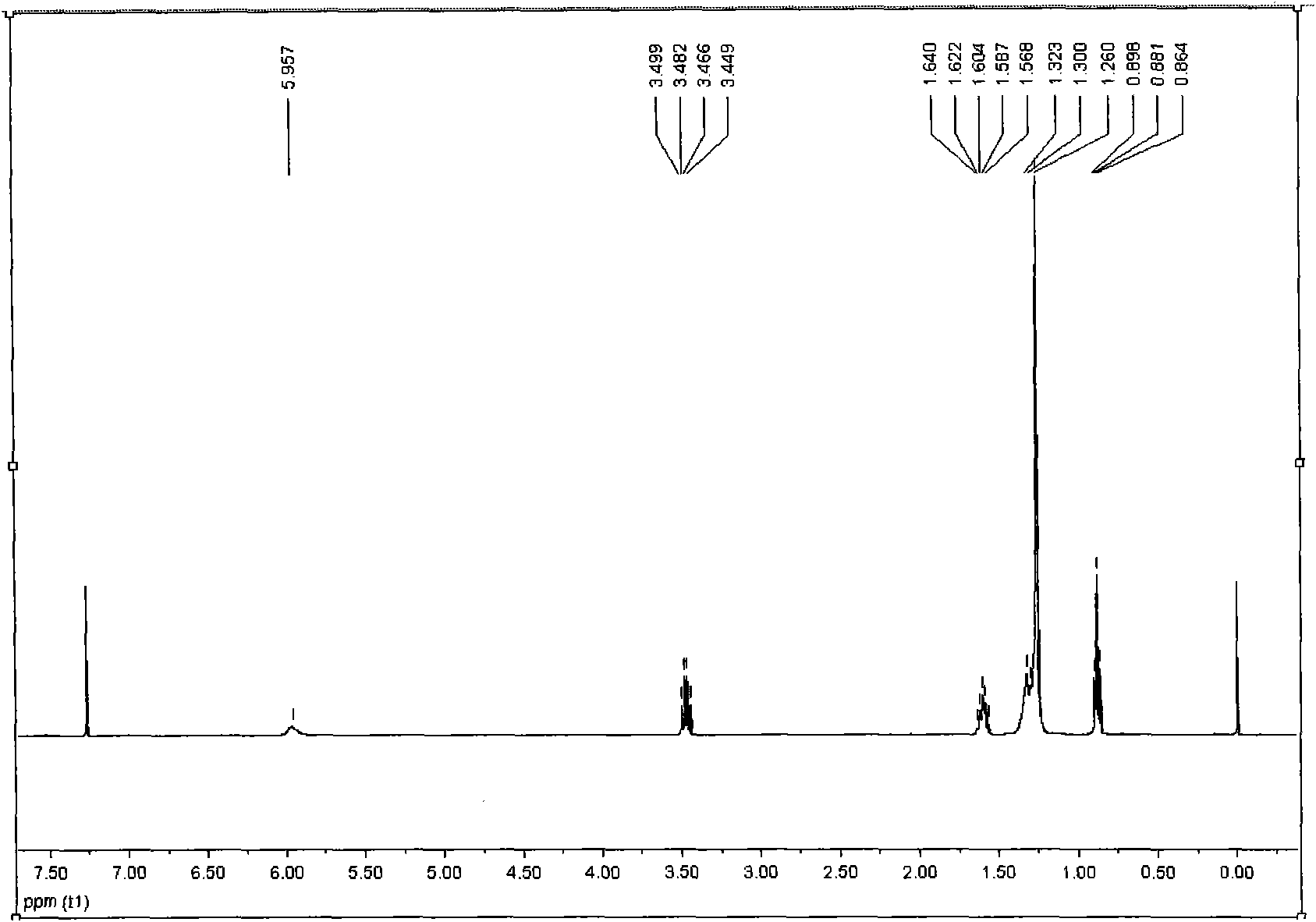
[0034] Add 1.84g (0.01mol) of cyanuric chloride and 8-12mL of acetone into the reaction flask, add dropwise 1.85g (0.01mol) of ethanol solution of dodecylamine at 0-5°C, and use 5-8% sodium hydroxide solution Adjust the pH to 7-10, and react at 0-5°C for 40 minutes. After completion of the reaction, remove acetone and ethanol, extract with ethyl acetate to obtain an ethyl acetate layer, remove ethyl acetate, and use eluent V 石油醚 / V 乙酸乙酯 Separation on a 7-10:1 column yielded 3.00 g of the intermediate 2-dodecylamino-4,6-dichloro-1,3,5-s-triazine with a yield of 90.4%. Such as figure 1 and figure 2shown.
[0035] (2) Synthesis of N-methyl-N-(4-dodecylamino-6-chloro-1,3,5-s-triazin-2-yl)-2-aminoethanesulfonate sodium amphoteric surfactant
[0036] Add 2.656g (0.008mol) of 2-dodecylamino-4,6-dichloro-1,3,5-s-triazine and 8-15mL of acetone into the reaction flask, and drop 3.22g (0.008mol) of...
Embodiment 2
[0040] (1) Synthesis of intermediate 2-decylamino-4,6-dichloro-1,3,5-s-triazine
[0041] Add 0.01mol cyanuric chloride and 10mL ethanol into the reaction flask, add dropwise 0.01mol decylamine in acetone solution at 0-5°C, adjust the pH to 8-11 with 8-10% sodium carbonate solution, and adjust the pH to 8-11 at 0-5°C. ℃ for 40 minutes. After completion of the reaction, remove acetone and ethanol, extract with ethyl acetate to obtain an ethyl acetate layer, remove ethyl acetate, and use eluent V 石油醚 / V 乙酸乙酯 Separation by a 7-10:1 column yielded 2.669 g of the intermediate 2-decylamino-4,6-dichloro-1,3,5-s-triazine with a yield of 87.80%.
[0042] (2) Synthesis of N-methyl-N-(4-decylamino-6-chloro-1,3,5-s-triazin-2-yl)-2-aminoethanesulfonate amphoteric surfactant
[0043] Add 2.58g (0.0085mol) of 2-decylamino-4,6-dichloro-1,3,5-s-triazine and 8-10mL of acetone into the reaction flask, and dropwise add 0.0085mol of 40% N-methyl Sodium taurine aqueous solution, adjust the pH to...
Embodiment 3
[0047] (1) Synthesis of 2-dodecylamino-4,6-dichloro-1,3,5-s-triazine intermediate
[0048] With embodiment 1 (1).
[0049] (2) Synthesis of N-methyl-N-(4-dodecylamino-6-chloro-1,3,5-s-triazin-2-yl)-2-aminoethanesulfonate sodium amphoteric surfactant
[0050] With embodiment 1 (2).
[0051] (3) Synthesis of N-methyl-N-(4,6-docosylamino-1,3,5-s-triazin-2-yl)-2-aminoethanesulfonate sodium amphoteric surfactant
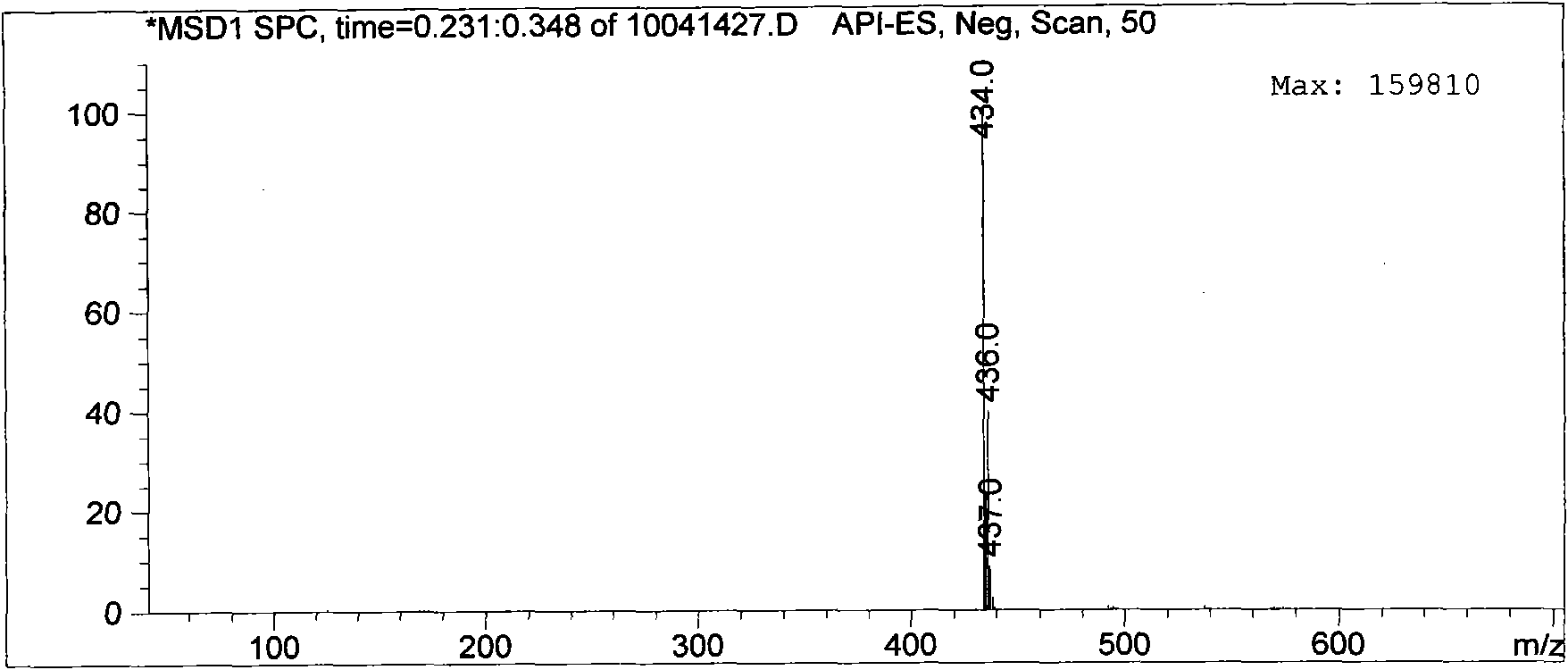
[0052] 2.742g (0.006mol) of N-methyl-N-(4-dodecylamino-6-chloro-1,3,5-s-triazin-2-yl)-2-aminoethanesulfonic acid sodium amphoteric Surfactant and 16mL ethanol aqueous solution were added to the reaction flask, 1.11g (0.006mol) ethanol solution of dodecylamine was added dropwise, the pH was adjusted to 8-10.5 with 5-8% sodium hydroxide solution, and the reaction was carried out at 90-95°C for 6 ~10h. After the reaction was completed, the reaction solution was concentrated under reduced pressure to precipitate a solid, which was filtered by suction and dried in vacuo to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com