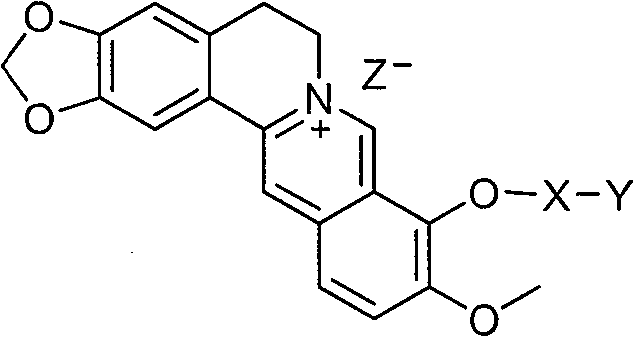
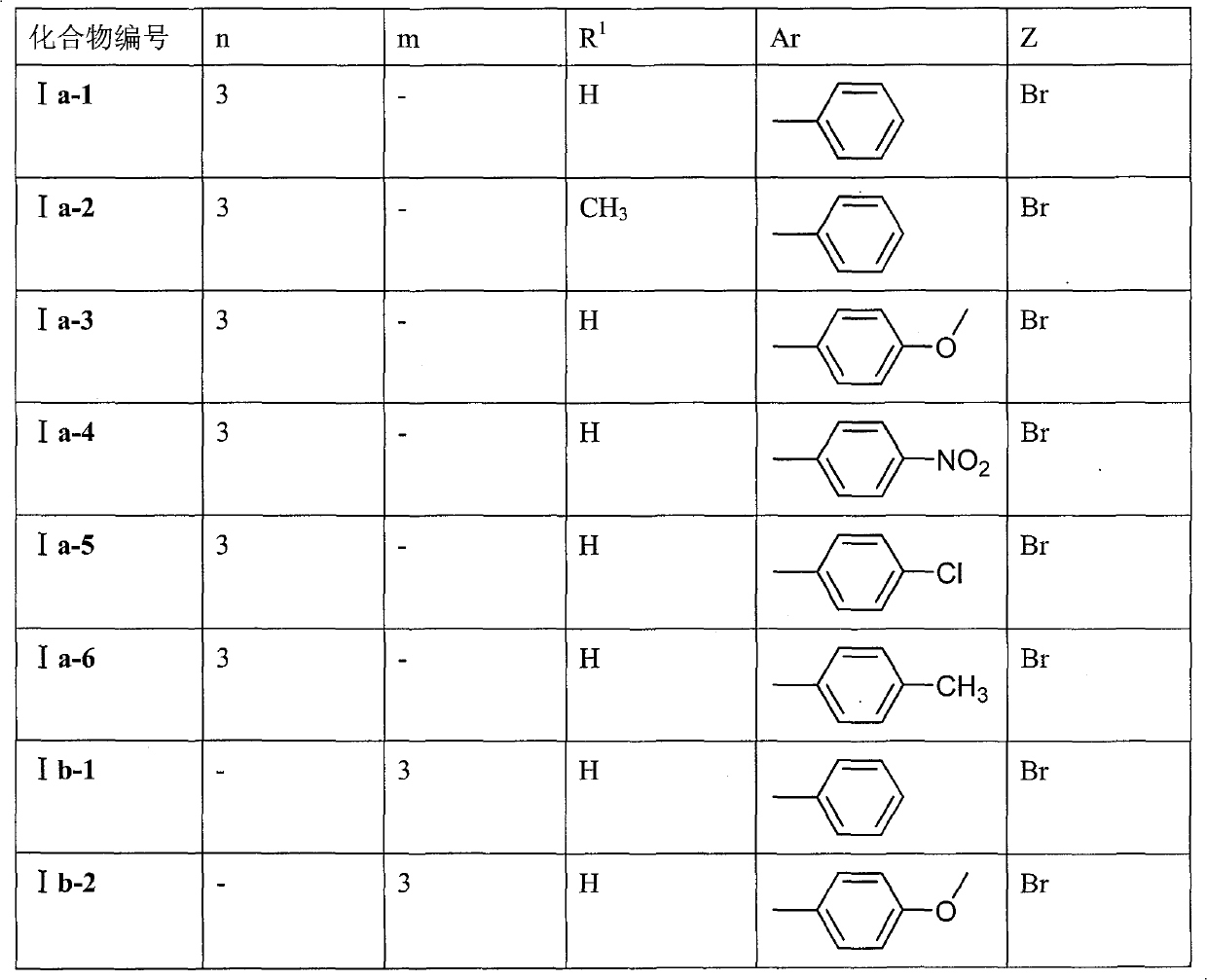
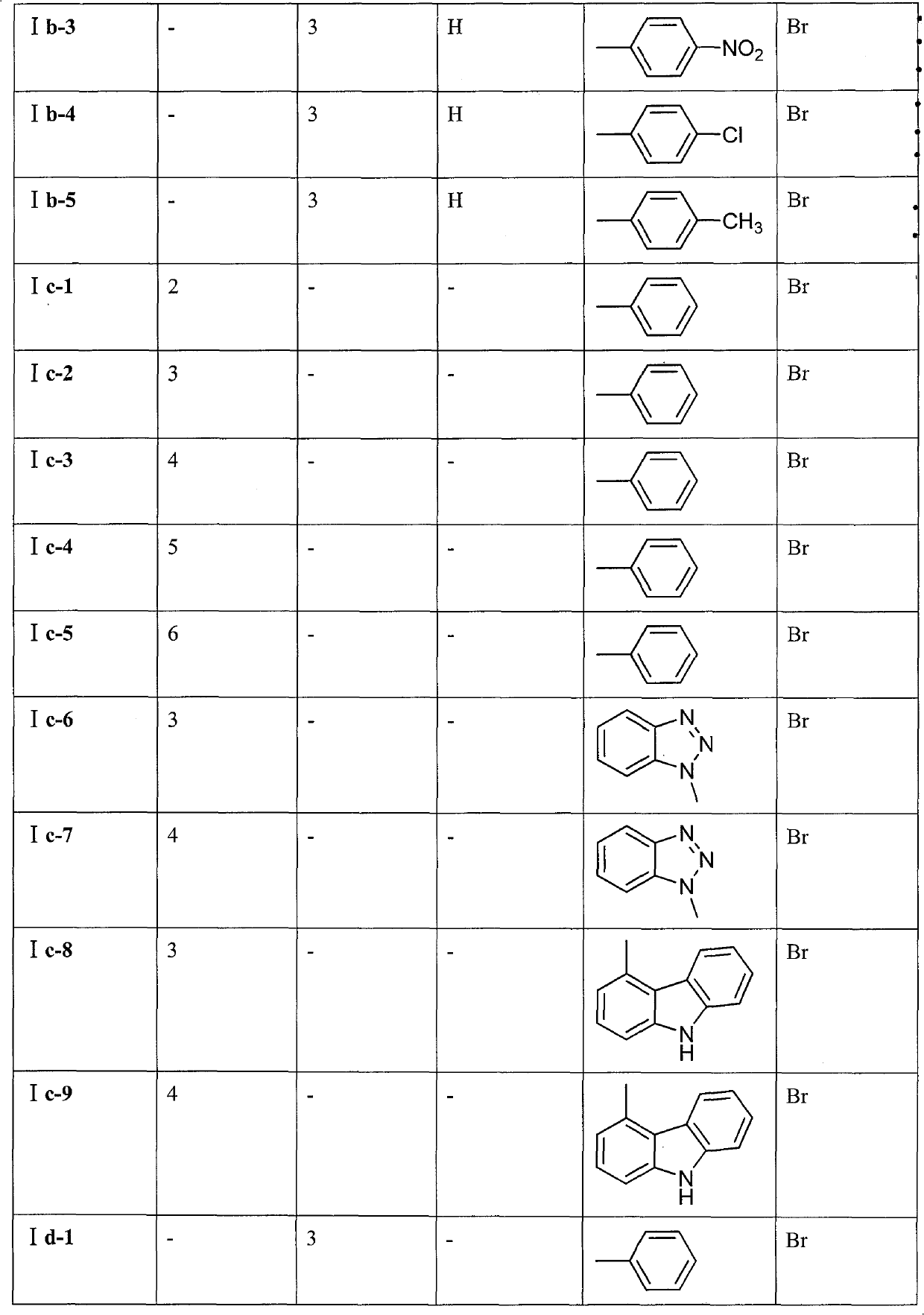
Preparation method and application of 9-bit substituent double-functional group berberine derivatives
A derivative, berberine technology, is applied in the field of preparation of 9-position substituted bifunctional berberine derivatives, can solve the problems of high liver toxicity, low bioavailability in vivo, etc., achieves less toxic side effects, inhibits Aβ Amyloid deposition, high therapeutic index effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1: Preparation 9-(3-(N-phenylamino) propoxy) berberine hydrobromide (Ia-1)
[0062] a step reaction:
[0063] Put 20mmol, 7.5g of dried berberine hydrochloride in a vacuum drying oven, control the pressure below 15mmHg, raise the temperature to 190°C, and react for 20min to obtain a dark red powder. The crude product is chromatographed with chloroform-methanol (10:1) After elution, compound 2 was obtained as bright red powder with metallic luster. Yield: 75%. 1 H NMR (400MHz, DMSO-d6) δ: 9.07(s, 1H), 8.94(s, 1H), 7.98(s, 1H), 7.61(s, 1H), 7.19(d, J=8.4, 1H), 6.96(s, 1H), 6.34(d, J 8.4, 1H), 6.10(s, 2H), 4.48(t, J 6.3.2H), 3.73(s, 3H), 3.04(t, J=6.4, 2H ); LC / MS (ESI) m / z: [M-Br]+321+.
[0064] Step b reaction:
[0065] Add aniline (10mmol, 0.933g), triethylamine (15mmol, 1.524g), 1,3-dibromopropane (12mmol, 2.423g) and 25ml of acetonitrile into a 50ml round bottom flask, and heat to reflux for 24h. After filtration and concentration, 1.75 g of the produ...
Embodiment 2
[0068] Embodiment 2: Preparation of 9-O-(3-N-phenylamino)-4-oxopropyl) berberine hydrobromide (Ib-1)
[0069] a step reaction:
[0070] With embodiment 1.
[0071] c-step reaction:
[0072] Add aniline (5mmol, 0.461g) and 25ml of anhydrous chloroform into a 50ml round bottom flask, add 3-bromopropionyl chloride (6mmol, 1.042g) and pyridine (6mmol, 0.480g) dropwise at 0°C, after the addition is complete , Stir the reaction at room temperature for 3h. The reaction solution was washed once with 30 ml of saturated sodium carbonate aqueous solution, 3 times with 30 ml of water, and once with saturated aqueous sodium chloride solution, and the organic phase was dried with anhydrous magnesium sulfate and filtered, and the filtrate was concentrated to obtain 3-bromo-N-phenylpropane The crude amide can be directly used in the next reaction.
[0073] f-step reaction:
[0074] Compound 2 (1mmol, 0.322g), potassium carbonate (5mmol, 0.700g), potassium iodide (0.1mmol, 0.016g) and cru...
Embodiment 3
[0075] Embodiment 3: Preparation 9-O-(2-(O-phenyl) ethyl) berberine hydrobromide (Ic-1)
[0076] a step reaction:
[0077] With embodiment 1.
[0078] d-step reaction:
[0079] Phenol (10mmol, 0.950g), potassium hydroxide (15mmol, 0.845g), 1,2-dibromoethane (12mmol, 2.221g) and butanone 25ml were added to a 50ml round bottom flask, and stirred at room temperature for 3h. After filtration and concentration, the product (2-bromoethoxy)phenyl was obtained by silica gel column chromatography 1.76g, yield: 88%. 1 H NMR (400MHz, CDCl 3 )δ: 7.17-7.23(m, 2H), 6.83-6.90(m, 3H), 4.39(t, J=6.0Hz, 2H), 3.81(t, J=6.4Hz, 2H).
[0080] f step reaction:
[0081] Compound 2 (1mmol, 0.322g), potassium carbonate (5mmol, 0.694g), potassium iodide (0.1mmol, 0.016g) and (2 bromoethoxy)phenyl (1.2mmol, 0.244g) were placed in a 50ml round bottom flask 30ml of anhydrous acetonitrile was added, and the reaction was heated under reflux for 12h. After filtration and concentration, 0.398 g of produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com