Mammary gland hyperplasia-treating capsule and preparation method thereof
A technology of soft capsules and capsule shells, which is applied in capsule delivery, pharmaceutical formulations, and medical preparations containing active ingredients, etc. It can solve the problems of capsule shell hardening and soft capsule instability, and achieve increased purity and stable product quality. good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
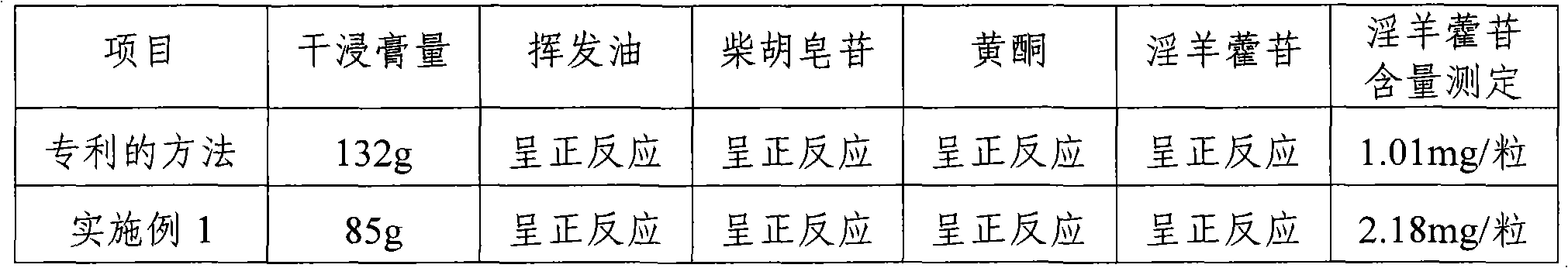
[0038] Embodiment 1: Ruzengning active ingredient extraction method
[0039] 1) Weigh Artemisia argyi, Epimedium, Bupleurum, Toosendan, Asparagus, and Fritillaria in a weight ratio of 2:1:1:1:1:1.2, mix them, add water to decoct, and add them for the first time Boil 10 times the amount of water for 1.5 hours; add 8 times the amount of water for the second time and boil for 1 hour; add 8 times the amount of water for the third time and boil for 1 hour, and the relative density of vacuum concentration is 1.28 (85°C);
[0040] 2) Stir while it is hot and add ethanol to make the alcohol concentration reach 70% (the amount of alcohol added is about three times the amount of the liquid medicine), continue to stir fully, let the alcohol precipitation solution stand for 24 hours, and take the supernatant;
[0041] 3) The precipitate was washed twice with a small amount of 70% ethanol and then merged with the supernatant, and the ethanol was recovered under reduced pressure to obtain the...
Embodiment 2
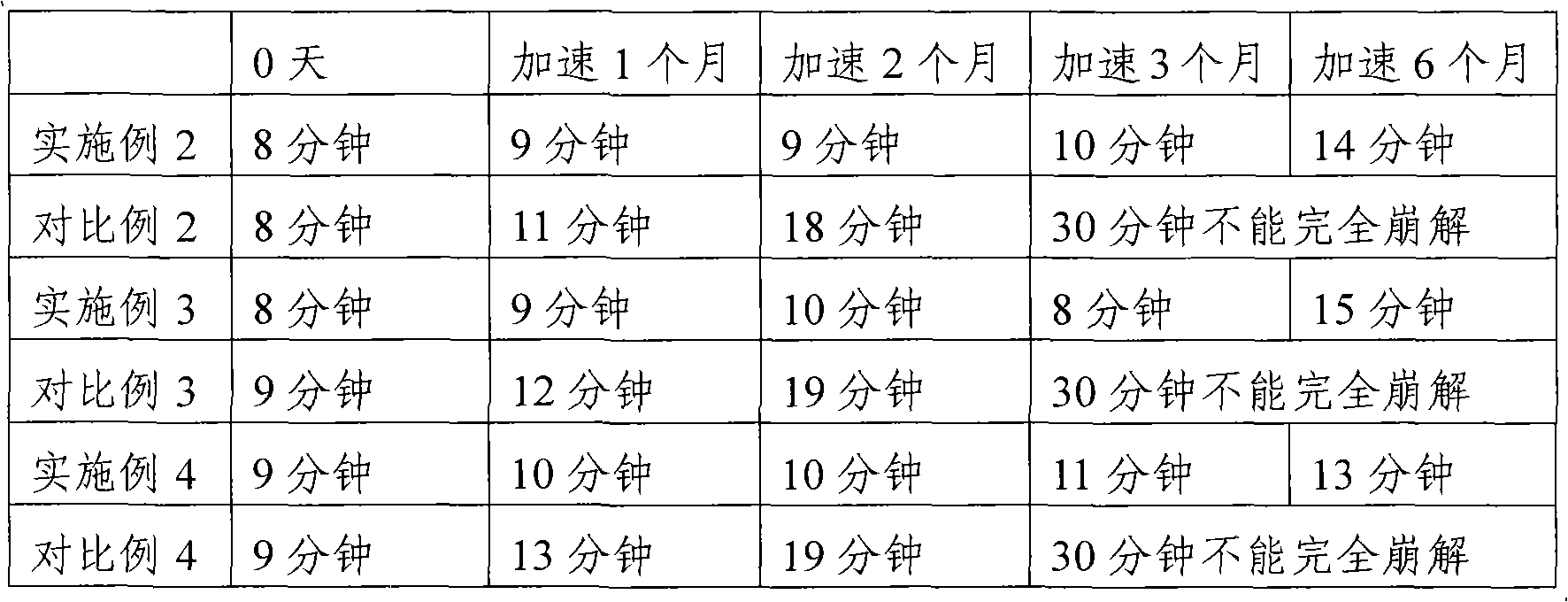
[0044] 1. Composition of soft capsules
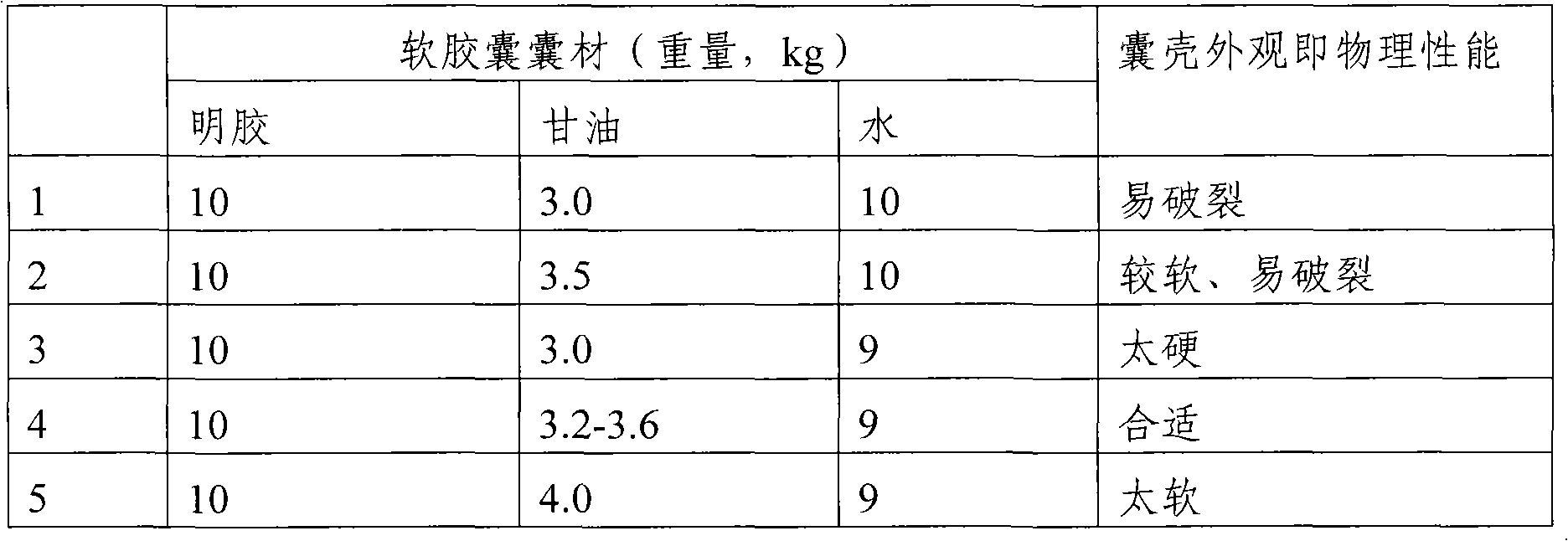
[0045] Wherein the composition of capsule shell: 10 parts of gelatin, 3.2 parts of glycerin, 9 parts of water (weight ratio).
[0046] 2, the concrete composition of soft capsule content is: wherein active ingredient adopts the method preparation of embodiment 1:
[0047] Content composition Weight (mg) Weight percentage (%)
[0048] Active ingredient (according to extract dry powder) 4 53.12
[0049] Polyethylene glycol 2000 3 39.84
[0050] Chitosan 0.25 3.32
[0051] Hydroxypropyl methylcellulose 0.13 1.73
[0052] Ascorbic acid 0.1 1.33
[0053] Methylparaben 0.05 0.66
[0054] Total 7.53 100
[0055] 3. The preparation method is:
[0056] 1) Preparation of the capsule shell liquid: According to the prescription ratio, mix the gelatin, glycerin and water of the capsule shell evenly, heat and dissolve in a water bath at 80°C, add an appropriate amount of methyl p-hydroxybenzoate, stir, vacuumize and degas, 70 ℃ heat preserva...
Embodiment 3
[0060] 1. The composition of the soft capsule, wherein the composition of the capsule shell: 10 parts of gelatin, 3.5 parts of glycerin, and 9 parts of water (weight ratio).
[0061] 2. The specific composition of the soft capsule content is: wherein the active ingredient is prepared by the method of Example 1
[0062] Content composition weight (mg) weight percentage (%)
[0063] Active ingredient (according to extract dry powder) 4 46.73
[0064] Polyethylene glycol 400 4 46.73
[0065] Glycerin 0.28 3.27
[0066] Hydroxypropyl methylcellulose 0.1 1.17
[0067] Ascorbic acid 0.14 1.63
[0068] Methylparaben 0.04 0.47
[0069] Total 8.56 100
[0070] 3. The preparation method is:
[0071] 1) Preparation of the capsule shell liquid: According to the prescription ratio, mix the gelatin, glycerin and water of the capsule shell evenly, heat and dissolve in a water bath at 80°C, add an appropriate amount of methyl p-hydroxybenzoate, stir, vacuumize and degas, 70 ℃ heat pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com