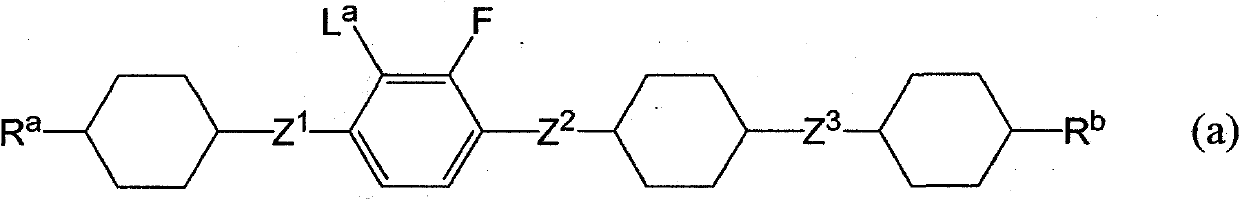
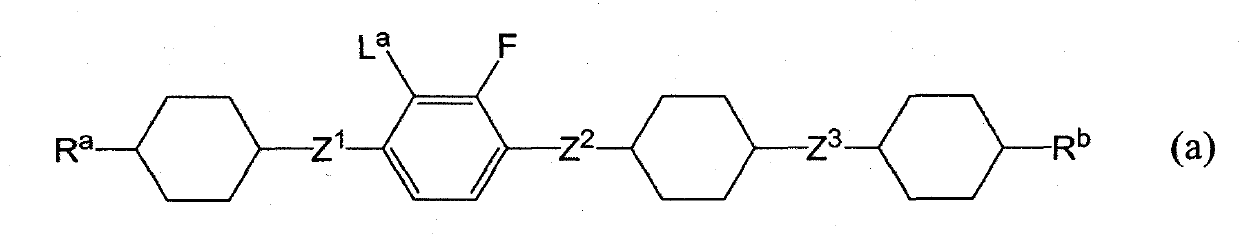
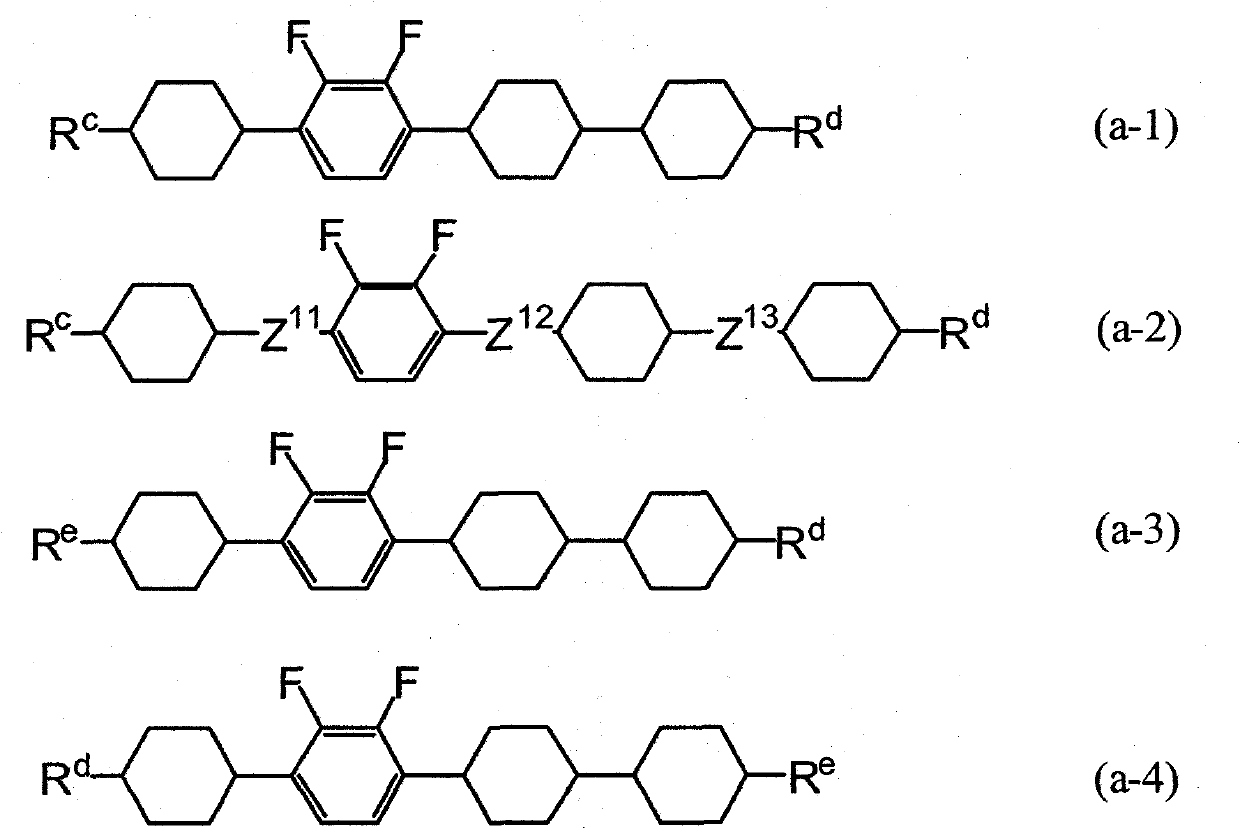
Liquid crystalline tetracyclic compound having fluorine atom, liquid crystal composition and liquid crystal display element
A technology of liquid crystal composition and compound, applied in liquid crystal materials, organic chemistry, nonlinear optics, etc., can solve the problems of insufficient temperature range, no transparent point, and high optical anisotropy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0215] The liquid crystal composition of the present invention is usually prepared by a known method, for example, a method capable of dissolving essential components at high temperature. In addition, additives well-known to those skilled in the art can be added according to the application to prepare, for example, the liquid crystal composition (e) of the present invention containing an optically active compound as described below, and the liquid crystal composition for GH mode to which a dye is added. In general, additives are well known to practitioners in this field and are described in detail in literature and the like.
[0216] The liquid crystal composition (e) of the present invention is a liquid crystal composition that further contains one or more optically active compounds in the above-mentioned liquid crystal composition of the present invention.
[0217] As an optically active compound, a known chiral dopant is added. The chiral dopant has the effect of making th...
Embodiment 1
[0252] Synthesis of 4-[2-fluoro-4-(4-pentyl-cyclohexyl)-phenyl]-4'-vinyl-bicyclohexane (No.5)
[0253]
[0254] Step 1
[0255] Add 56.2 g of 1-fluoro-3-(4-pentyl-cyclohexyl)-benzene (1) and 500 ml of tetrahydrofuran (THF) into the reactor under nitrogen atmosphere, and then cool to -73°C. Thereto, 250.0 ml of 1.04 M 2-butyllithium n-hexane and cyclohexane solutions were added dropwise at a temperature ranging from -74°C to -65°C, followed by further stirring for 30 minutes. Then, 57.2 g of 4-(1,4-dioxaspiro[4,5]decane-8-yl)cyclohexanone (2) was dissolved by dropwise addition within the temperature range of -76°C to -65°C The solution formed in 160 ml of THF was then returned to 25°C and stirred for a further 20 hours. Thereafter, the reaction mixture was poured into a container containing 27.8 g of ammonium chloride and 1000 ml of ice water, and mixed. 1360ml of toluene was added, and the extraction operation was performed after it was separated into an organic layer an...
Embodiment 2
[0277] Synthesis of 4-(2-fluoro-4-(4-pentylcyclohexyl)phenyl)-4'-vinylbicyclohexane (No.3)
[0278]
[0279] Using 1-fluoro-3-(4-propylcyclohexyl)benzene (11) instead of 1-fluoro-3-(4-pentyl-cyclohexyl)-benzene (1), in the same way as in Example 1 Synthesis of 4-(2-fluoro-4-(4-pentylcyclohexyl)phenyl)-4'-vinylbicyclohexane (No. 3).
[0280] 1 The chemical shift δ (ppm) of H-NMR analysis is as follows, and the obtained compound can be identified as 4-(2-fluoro-4-(4-pentylcyclohexyl)phenyl)-4'-vinylbicyclohexyl (No. 3). In addition, the assay solvent is CDCl 3 .
[0281] Chemical shift δ (ppm): 7.11(dd, 1H), 6.91(dd, 1H), 6.84(dd, 1H), 5.78(m, 1H), 4.96(m, 1H), 4.88(m, 1H), 2.74 (m, 1H), 2.42(m, 1H), 1.86-1.79(m, 13H), 1.45-0.99(m, 19H), 0.90(t, 3H).
[0282] The transition temperature of the obtained compound (No. 3) is as follows.
[0283] Transition temperature: C 41.9 C 76.3 S B 168.2 N 303.5 I
[0284] Physical properties of compound (No.3)
[0285] Prepare 4-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com