Tetrahydroisoquinoline derivative, preparation method and application thereof
A technology of tetrahydroisoquinoline and tetrahydroisoquinoline hydrochloride, applied in the field of tumor multidrug resistance reversal agent, tetrahydroisoquinoline derivatives and pharmaceutically acceptable salts, can solve the problem of specific action Weakness, cardiovascular side effects, high fat solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
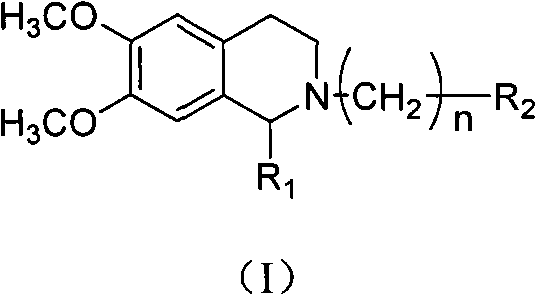
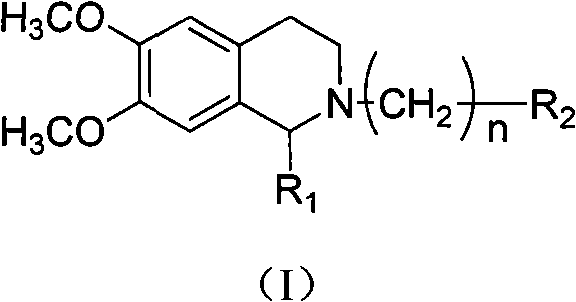
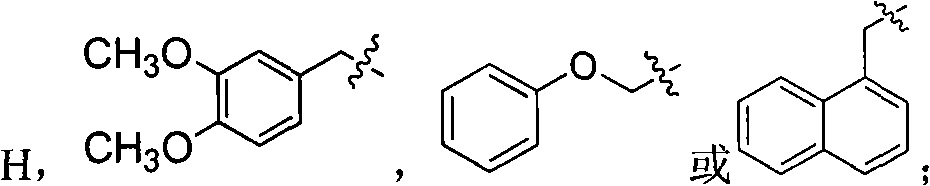
[0048] Embodiment: according to tetrahydroisoquinoline derivatives of the present invention, its general formula I compound can be prepared according to the following method:
[0049] (1) The compound of general formula I can be prepared according to the following method: 3,4-dimethoxyphenethylamine (compound 2) is reacted with substituted acetic acid under 190 DEG C of solvent-free compound 3, and compound 3 is prepared in trichloroxy In the presence of phosphorus, reflux in anhydrous toluene to obtain compound 4, compound 4 is under the condition of anhydrous methanol as a solvent, add a catalytic amount of diethylamine, and be reduced by potassium borohydride to obtain compound 5, compound 5 and oxadiazole (II) In acetonitrile, use anhydrous potassium carbonate as an acid-binding agent to reflux for 2 to 8 hours to obtain compound I, compound 5 and substituted phenoxyalkyl bromide (III) in acetonitrile, use anhydrous potassium carbonate as an acid-binding agent to reflux for...
Embodiment 1
[0112] Example 1: 6,7-dimethoxy-3,4-dihydroisoquinoline (4a)
[0113] Mix 40g (0.22mol) of 3,4-dimethoxyphenethylamine with 18.4g of 36% (0.22mol) formaldehyde, gradually heat up to 100°C, reflux for 30 minutes, stop heating, and after a little cooling (yellow oil-water mixture) pipette Suck off the upper aqueous solution, then add 4 times the amount of 23% hydrochloric acid, evaporate the reaction solution to dryness to obtain a brownish yellow solid, recrystallize with 200ml of 95% ethanol to obtain 35g of white needle crystals, add 45ml of concentrated ammonia water to the crystals, Slightly heated until completely dissolved without viscous matter, extracted with dichloromethane, washed with saturated brine until neutral, dried over anhydrous sodium sulfate, filtered and evaporated to dryness to obtain 28.5 g of yellow solid powder, yield: 67.9%, which was directly used in the following step response.
Embodiment 2
[0114] Example 2: 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (5a)
[0115] 28.5g (0.15mol) of compound 4a was dissolved in 160ml of methanol, 16g (0.38mol) of potassium borohydride was added in batches under an ice-water bath, and stirred at room temperature for 22h. The reaction solution was slowly poured into 500ml of ice-cold brine, and allowed to stand overnight to fully hydrolyze the potassium borohydride. Extracted with dichloromethane (100ml×3), washed with saturated brine until neutral, dried over anhydrous sodium sulfate, filtered and evaporated to dryness to obtain 26.1g of white solid 5a, yield: 92%. mp: 74-76°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com