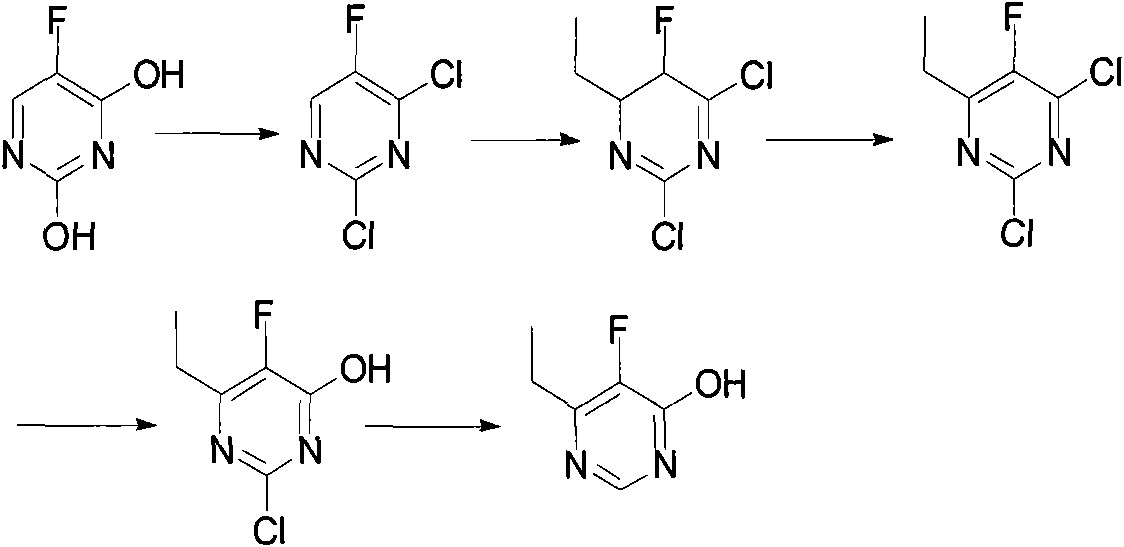
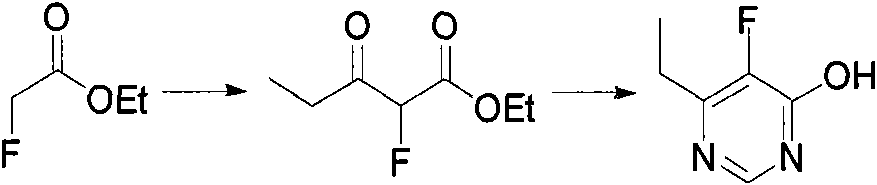
Method for synthesizing 6-ethyl-5-fluoro-4-hydroxy pyrimidine and intermediate thereof
A technology of hydroxypyrimidine and intermediate, applied in the field of chemical synthesis, can solve the problems of many three wastes, high cost, complicated process and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0009] Preparation of embodiment 1.2-fluoro-3-oxopentanoic acid ethyl ester
[0010] N 2 Add 1.2 L of isopropyl ether to a 2 L three-necked flask under protection, add about 44 g of sodium hydrogen, and stir evenly at room temperature. 106 g of ethyl fluoroacetate was slowly added dropwise into the reaction bottle, and the dropwise addition was completed within 3 hours. After the reaction system was cooled to 0°C, 95 g of propionyl chloride was slowly added dropwise, and the addition was completed in 3 hours. The temperature was controlled at 0-5°C, and the reaction was completed after stirring at this temperature for about 12 hours. 0.5 L of ice water was added under thorough stirring, the reaction system was adjusted to neutral with 5% NaOH solution, and the mixture was allowed to stand to separate layers. The aqueous phase was extracted three times with 500 ml isopropyl ether, the organic phases were combined, dried over anhydrous sodium sulfate, and the solvent was remov...
Embodiment 2
[0011] The preparation of embodiment 2.6-ethyl-5-fluoro-4-hydroxypyrimidine
[0012] N 2 Add 300ml of methanol to a 1L three-neck flask under protection, and after cooling down to 0°C, put 35g of sodium methoxide into the reaction flask, stir evenly, and keep the temperature in the bottle at 0-5°C. Add 33.4 grams of methyl ether to the bottle, stir at 0-5°C for about 1 hour, then slowly add 52 grams of ethyl 2-fluoro-3-oxopentanoate (dissolved in 20ml of methanol) dropwise into the bottle , The addition is completed within 1 hour, and the temperature in the bottle is maintained at 0-5°C. Remove the ice bath, slowly rise to room temperature, continue to stir and react for 24 hours, then add 15.4 g of glacial acetic acid to the bottle, adjust the pH to 6-6.5, and stir evenly. The solvent methanol was distilled off under reduced pressure to obtain an off-white solid mixture, which was fully extracted 5 times with dichloromethane (100ml×5), the organic phases were combined, the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| nuclear magnetic resonance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com