Medical use of (S)-3-fluoro-2-(4-isobutyl phenyl) propionic acid
A kind of technology of isobutylbenzene and propionic acid, applied in the field of pharmacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 Synthesis of (S)-3-fluoro-2-(4-isobutylbenzene)propionic acid
[0019]
[0020] In a round bottom flask, combine optically pure (R)-1-(1-fluoro-3-enyl-2-butyl)-4-isobutylbenzene (41.7 mg) and sodium periodate (213.8 mg) ) Was dissolved in 2 ml of a mixed solvent of carbon tetrachloride and acetonitrile (1:1), then an aqueous solution (1.5 ml) of hydrated ruthenium trichloride (2.1 mg) was added, stirred at room temperature for 1.5 hours, and ether (5 ml ) And saturated aqueous sodium bicarbonate solution (5 mL). Extract with aqueous sodium bicarbonate solution (5 mL x 5), combine the aqueous phases, slowly add concentrated hydrochloric acid at zero degrees Celsius to adjust the pH to 1, then extract with dichloromethane (5 mL x 4), combine the organic phases, and anhydrous After drying over sodium sulfate, the solvent was removed under reduced pressure to obtain the target product (S)-3-fluoro-2-(4-isobutylbenzene)propionic acid. White solid, 91% yield, [α] D 2...
Embodiment 2
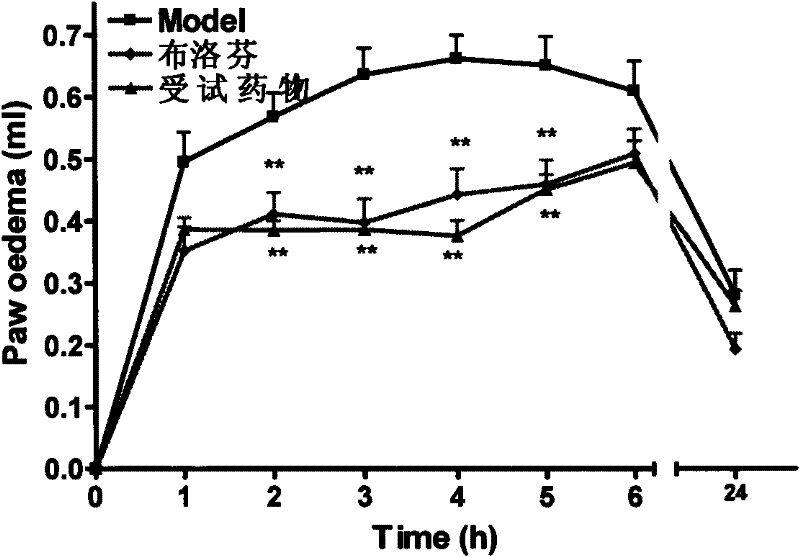
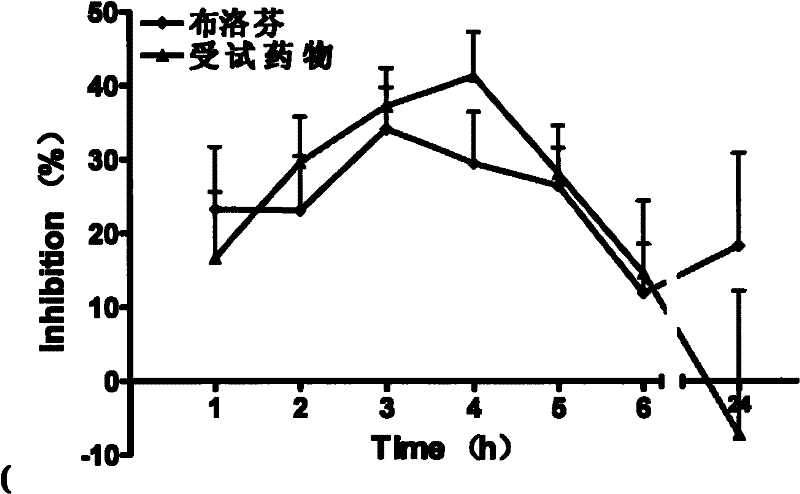
[0021] Example 2 Anti-inflammatory effect of (S)-3-fluoro-2-(4-isobutylbenzene)propionic acid
[0022] Model selection: carrageenan-induced acute inflammation model of rat foot swelling
[0023] Basic principle: Carrageenan increases the synthesis of prostaglandin (PG) in the inflamed area, and induces edema together with vasoactive amines such as histamine, 5-HT and kinins.
[0024] Sample preparation:
[0025] Positive control drug: ibuprofen
[0026] Preparation method: Dissolve and dilute with 0.5% CMC into a white turbid liquid.
[0027] Drug administration dose: 30mg / kg
[0028] Drug administration volume: 1ml / 100g
[0029] Drug administration method: i.g.
[0030] Test drug: (S)-3-fluoro-2-(4-isobutylbenzene)propionic acid
[0031] Preparation method: Dissolve and dilute with 0.5% CMC to a pale yellow turbid liquid.
[0032] Drug administration dose: 33.6mg / kg
[0033] Drug administration volume: 1ml / 100g
[0034] Drug administration method: i.g
[0035] Carrageenan (λCarrageenan) was pur...
Embodiment 3
[0058] Example 3 Analgesic effect of (S)-3-fluoro-2-(4-isobutylbenzene)propionic acid
[0059] Model selection: chemical stimulation method
[0060] Basic principle: Many chemical substances, such as strong acids, strong bases, potassium ions, bradykinin, etc., cause pain when they come into contact with intact skin and mucous membranes. Therefore, certain chemical substances can be injected into animals to produce pain and cause pain models, which are used as methods for studying pain physiology and screening analgesic drugs. The mouse writhing method is one of the commonly used methods for screening analgesics. Some chemical stimulants are injected into the abdominal cavity of mice, causing deep, large-area and longer-lasting painful stimulation, causing the mice to have a "writhing" response ( The abdomen is recessed, the trunk and hind legs are stretched, and the buttocks are raised). Observe the number of "writhing" in mice that have a "writhing" reaction within 10 minutes o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com