Method for synthesizing veratraldehyde
A technology of veratraldehyde and its mixture, which is applied in the field of synthesizing veratraldehyde, can solve the problems of difficult popularization and application, high cost, high price of vanillin, etc., achieve good economic and social value, reduce cost, and shorten the process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
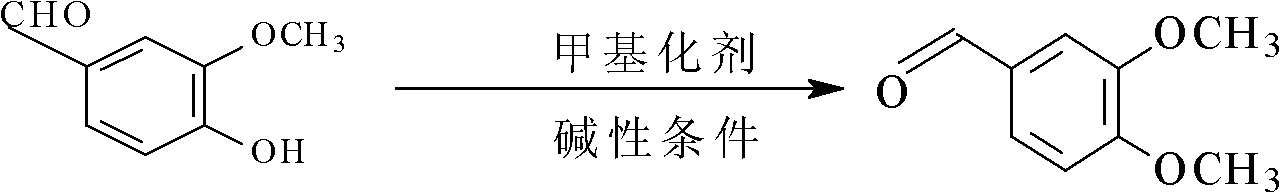
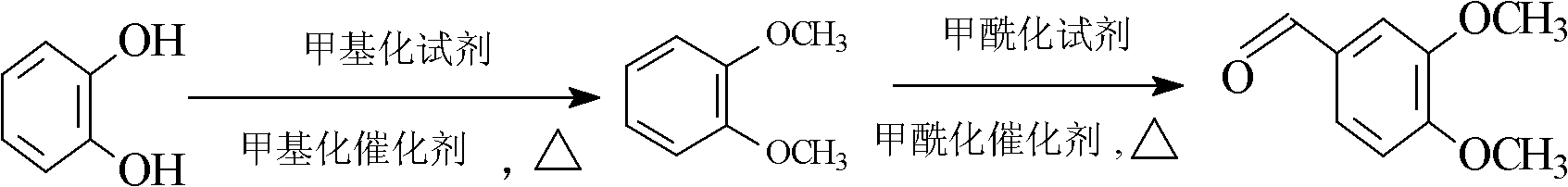
[0029] A method for synthesizing veratraldehyde, characterized in that: using catechol as raw material, undergoing methylation reaction and formylation reaction successively, catechol generates veratraldehyde after the above-mentioned two-step reaction; wherein:
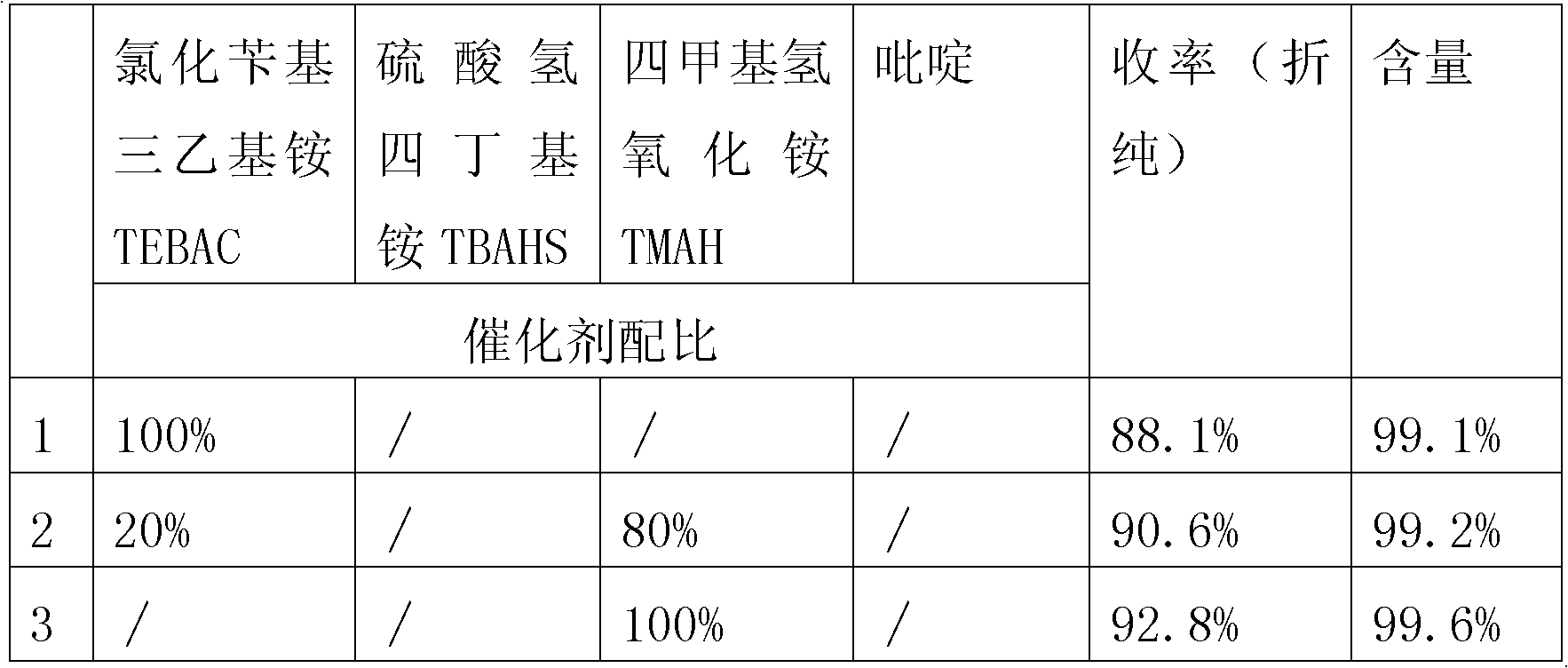
[0030] (1) Catechol synthesis of veratrole: add catechol 1100g in 5000mL reactor A, dissolve catechol with toluene solvent, add dimethyl sulfate 2400g to catechol, heat The mixture formed by catechol and dimethyl sulfate, the initial temperature is controlled at 20-40°C, 11.0g methylation catalyst benzyltriethylammonium chloride (TEBAC) is dissolved with 1200mL liquid caustic soda (30%wt) , metered and dropped 830mL of the above mixed solution under the condition of stirring, and controlled the rate of addition to complete the dropwise addition within 5 hours. During the dropping process, the temperature of the reaction solution was controlled at 35-40°C, and continued to stir for 30min after the dropwise addition; ca...
Embodiment 2
[0035] A method for synthesizing veratraldehyde, characterized in that: using catechol as raw material, undergoing methylation reaction and formylation reaction successively, catechol generates veratraldehyde after the above-mentioned two-step reaction; wherein:
[0036](1) Catechol synthesis of veratrole: add catechol 1100g in 5000mL reactor A, dissolve catechol with toluene solvent, add dimethyl sulfate 2400g to catechol, heat The mixture formed by catechol and dimethyl sulfate, the initial temperature is controlled at 20-40°C, the methylation catalyst is benzyltriethylammonium chloride (TEBAC) 2.2g, tetramethylammonium hydroxide (TMAH) 8.8g was dissolved with 1200mL liquid caustic soda (30%wt), and 830mL of the above-mentioned mixed solution was metered and dropped under stirring conditions, and the dropping rate was controlled to finish adding in 5 hours. During the dropping process, the temperature of the reaction solution was controlled at 35-40°C, Continue to stir for 3...
Embodiment 3
[0039] A method for synthesizing veratraldehyde, using catechol as raw material, successively undergoes methylation reaction and formylation reaction, and catechol generates veratraldehyde after the above-mentioned two-step reaction; wherein:
[0040] (1) pyrocatechol is synthesized veratrole: in 6300L reactor A, add pyrocatechol 1100Kg, at least one solvent 200kg in toluene, butyl acetate, acetone is wetting and dissolving pyrocatechol; Add Dimethyl sulfate 2018kg, be stirred into slurry; Tetramethylammonium hydroxide (TMAH) 10kg, dissolve with 3500L liquid caustic soda (30%wt), measure and drop above-mentioned mixed solution 1500L under stirring condition, control drop rate in 5 hours After the dropwise addition, control the temperature of the reaction solution at 20-40°C, and continue to stir for 30 minutes after the dropwise addition; under the action of the catalyst, catechol and methylating reagent are partially converted into guaiacol; To 70°C, continue to drop the rema...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com