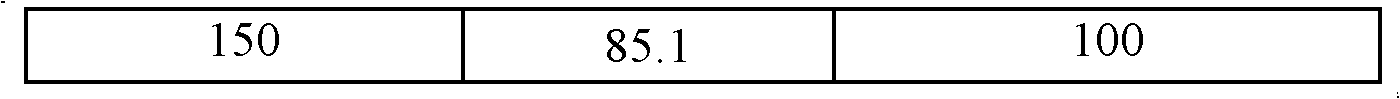Method for preparing S-(+)-3-hydroxy tetrahydrofuran through microbial conversion
A technology for the conversion of hydroxytetrahydrofuran and microorganisms, which is applied in the direction of fermentation, etc., can solve the problems of cumbersome preparation process of chiral catalysts, difficult separation and extraction, and unsuitability for industrial production, so as to achieve easy large-scale industrial production, large-scale cultivation, The effect of increasing the molar conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Inoculate CGMCC No.2266 bacteria into the slant medium, and culture at 30°C for 4-6 days to obtain the slant of bacteria. Use an inoculation needle to take an inoculation loop from the slant of the bacterium and inoculate it into a 250mL Erlenmeyer flask containing 100mL of liquid medium, and incubate at 30°C and 180r / min for 24h to obtain a seed solution. Inoculate 10 mL of seed solution (inoculum volume fraction 10% of the medium) into a 250 mL Erlenmeyer flask containing 100 mL of liquid medium, cultivate it for 24 hours at 30°C and 180 r / min to obtain a fermented liquid, centrifuge the fermented liquid, Obtain enzyme-containing bacterial cells.
[0035] The determination of the dry weight of the bacteria is to take a small part of the wet bacteria containing the enzyme cells after centrifuging the fermentation broth, and dry it at 120°C for 48 hours to a constant weight, measure the weight of the dry cells, and calculate the unit content of the fermentation broth. ...
Embodiment 2
[0041] Inoculate CGMCC No.2266 bacteria into the slant medium, and culture at 30°C for 4-6 days to obtain the slant of bacteria. Use an inoculation needle to take an inoculation loop from the slant of the bacterium and inoculate it into a 250mL Erlenmeyer flask containing 100mL of liquid medium, and incubate at 30°C and 180r / min for 24h to obtain a seed solution. Inoculate 10 mL of seed solution (the inoculation amount is based on 10% of the volume of the medium used) in a 250 mL Erlenmeyer flask containing 100 mL of liquid medium, cultivate it for 24 hours at 30°C and 180 r / min to obtain a fermentation liquid, and centrifuge the fermentation liquid to obtain Enzyme-containing bacterium cells, the dry weight of enzyme-containing bacterium cells per liter of fermentation broth is 50 grams.
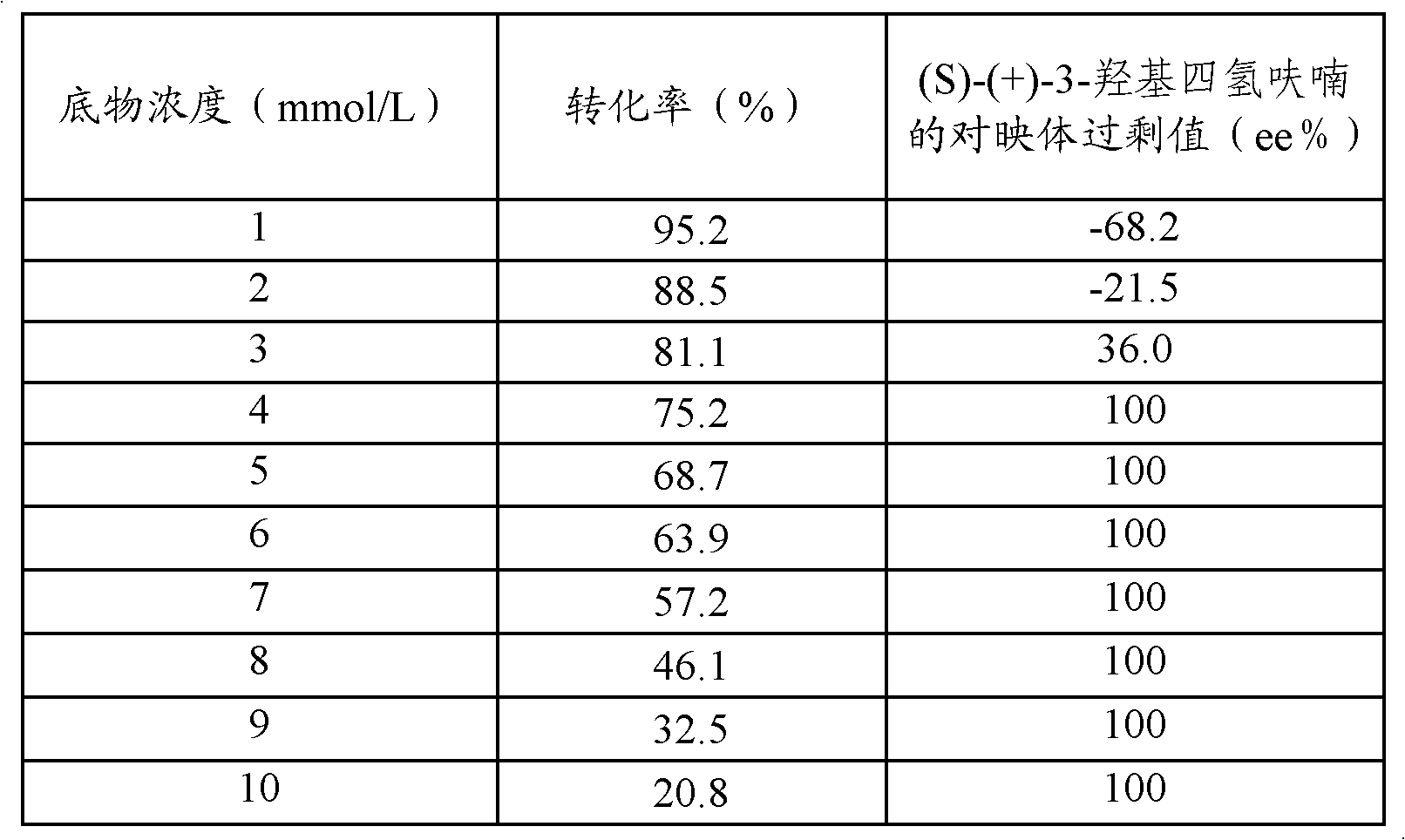
[0042] Add the wet cells obtained after centrifugation of the above 200 ml fermentation broth to ten portions of Erlenmeyer flasks each containing 100 mL pH7.0 phosphate buffer solution, wh...
Embodiment 3
[0048] Saccharomyces cerevisiae CGMCC No.2266 strain was inoculated into the slant medium, and cultured at 30° C. for 4 to 6 days to obtain the slant surface of the bacteria. Use an inoculation needle to take an inoculation loop from the slant of the bacterium and inoculate it into a 250mL Erlenmeyer flask containing 100mL of liquid medium, and incubate at 30°C and 180r / min for 24h to obtain a seed solution. Inoculate 10 mL of seed solution (the inoculation amount is based on 10% of the volume of the medium used) in a 250 mL Erlenmeyer flask containing 100 mL of liquid medium, cultivate it for 24 hours at 30°C and 180 r / min to obtain a fermentation liquid, and centrifuge the fermentation liquid to obtain Enzyme-containing bacterium cells, the dry weight of enzyme-containing bacterium cells per liter of fermentation broth is 50 grams.
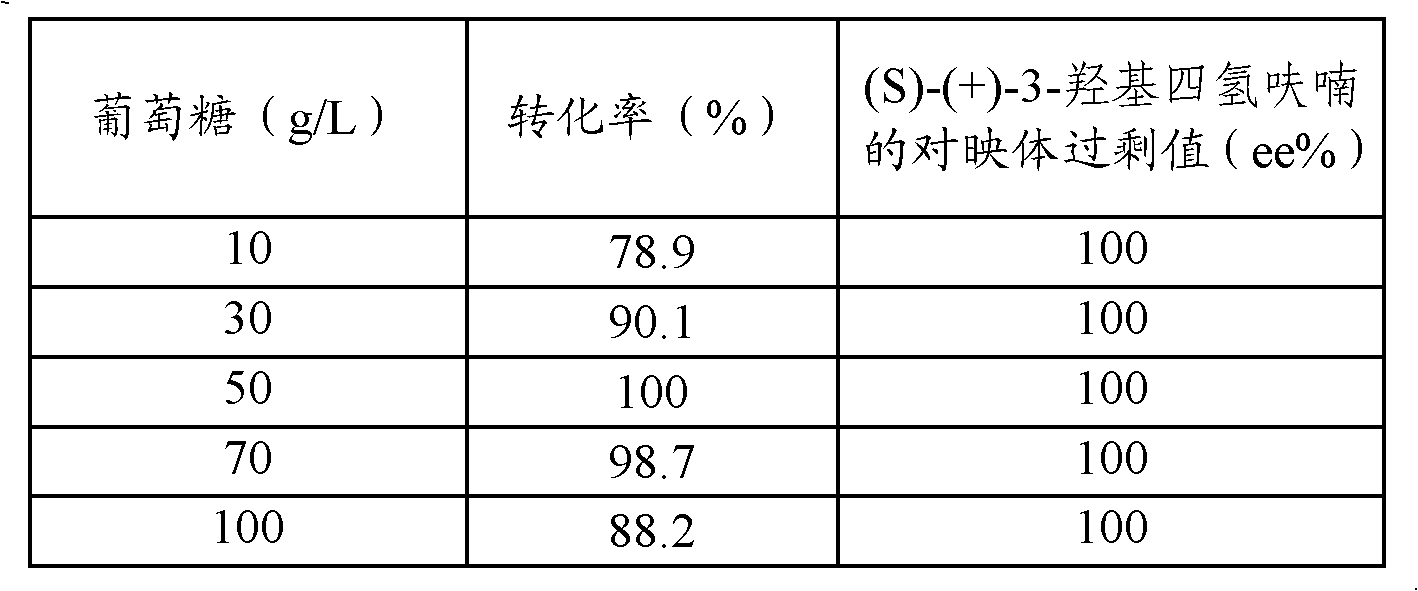
[0049] Add the wet cells obtained after centrifugation of the above-mentioned 200 milliliters of fermentation broth to five parts of Erlenmeyer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com