Process for high-efficiency expressing sTNFR/Fc fusion protein in yeast and uses thereof
A fusion protein and high-efficiency expression technology, which is applied to peptide/protein components, microbial-based methods, and medical preparations containing active ingredients. Low cost, easy to cultivate, fast propagation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: High expression of human sTNFRII / Fc in Pichia pastoris
[0046] 1. Construction of expression vector
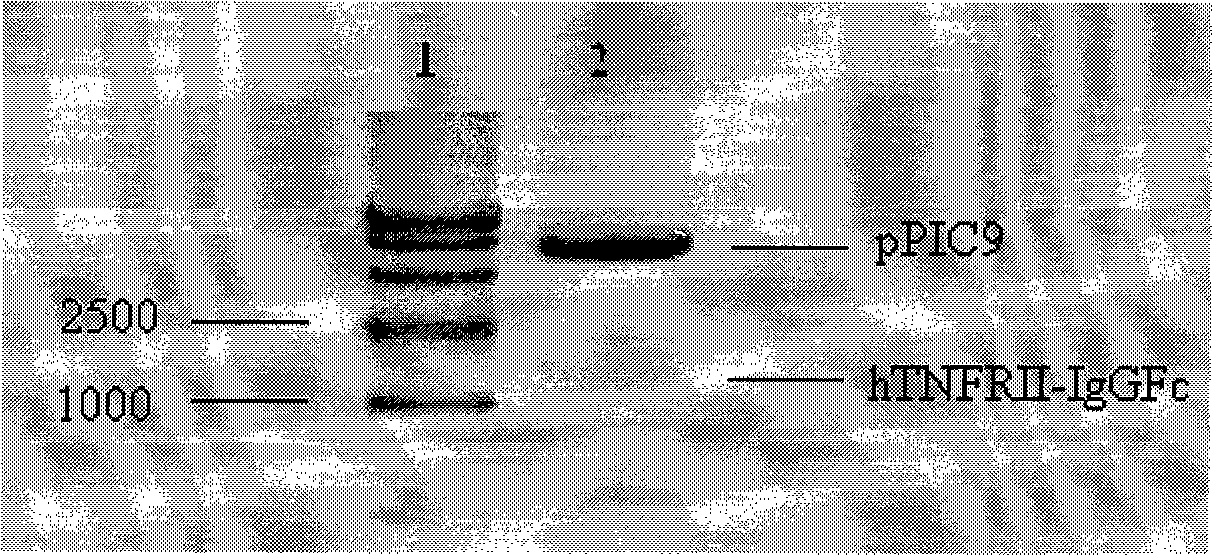
[0047] Take acid citrate dextrose (ACD) anticoagulated venous blood from healthy people, dilute with Hanks solution, centrifuge lymphocytes with lymphocyte separation medium, and use 1640 medium (GIBCO) containing 10% fetal bovine serum (Hyclone company) Adjust the number of cells to 5 × 10 6 cells / mL, placed in a cell incubator for 2 hours and then replaced with fresh medium containing 1640 ester polysaccharide (LPS, 20 μg / mL) (Sigma) and 10% fetal bovine serum, and continued to culture for 5 hours to enrich cells by centrifugation; Total RNA was extracted by TRIzol (Shanghai Sangon Bioengineering Technology Service Co., Ltd.) method (operate according to TRIzol instructions); cDNA was obtained from total RNA after RT-PCR (operate according to RT-PCR kit instructions), and then used as Template, using primers N1 / C1, N2 / C2, pfu polymerase (TaKaRa) PCR to c...
Embodiment 2
[0051] Example 2: High expression of optimized human sTNFRII / Fc fusion gene in Pichia pastoris
[0052] 1. Gene optimization and synthesis
[0053] On the premise of not changing the amino acid sequence, the DNA sequence of sequence 3 was designed by replacing the codons in sequence 2 with yeast preferred codons, optimizing the secondary structure of mRNA, and reducing the hairpin structure. In order to synthesize the gene, we adopt the method of segmented synthesis and PCR splicing. That is, the DNA forward fragments of sequence 3 were first synthesized, each segment was 58bp, and its reverse complementary sequence was 58bp / segment, and each reverse segment was complementary to the previous segment and the subsequent segment of the forward sequence by 29 bp respectively. (Shanghai Sangon Bioengineering Technology Service Co., Ltd.). Get 1 pmol of all DNA fragments, mix, use primer 1: ATCTCGAGAAAAGAGCTTTGCCAGCTCAAGTTGCTTTCA (SEQID No.8) and primer 2: ACGAATTCTCTAGATCATTACTTA...
Embodiment 3
[0057] Example 3: High-efficiency expression of sTNFRII / Fc fusion protein in α-1,6-mannosyltransferase-deficient yeast
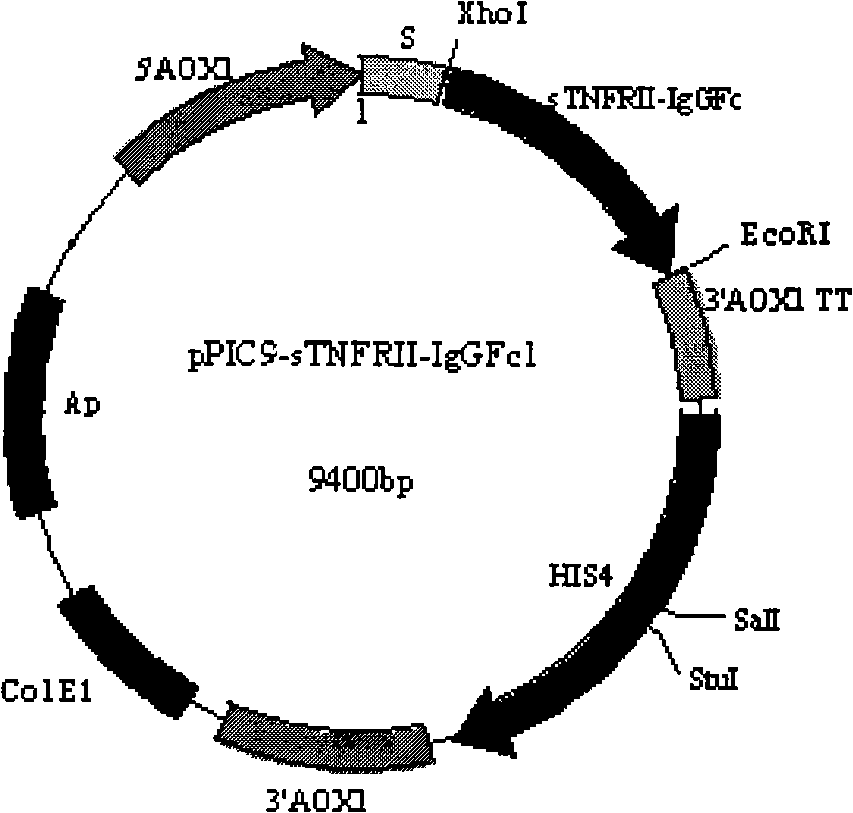
[0058] In this example, the Pichia pastoris GS115 (China Culture Collection Center, accession number CGMCC NO.1853) expression system after knockout of the α-1,6-mannosyltransferase (Ochlp) gene was used to express the TNF fusion protein. pPIC9 was used as the expression vector.
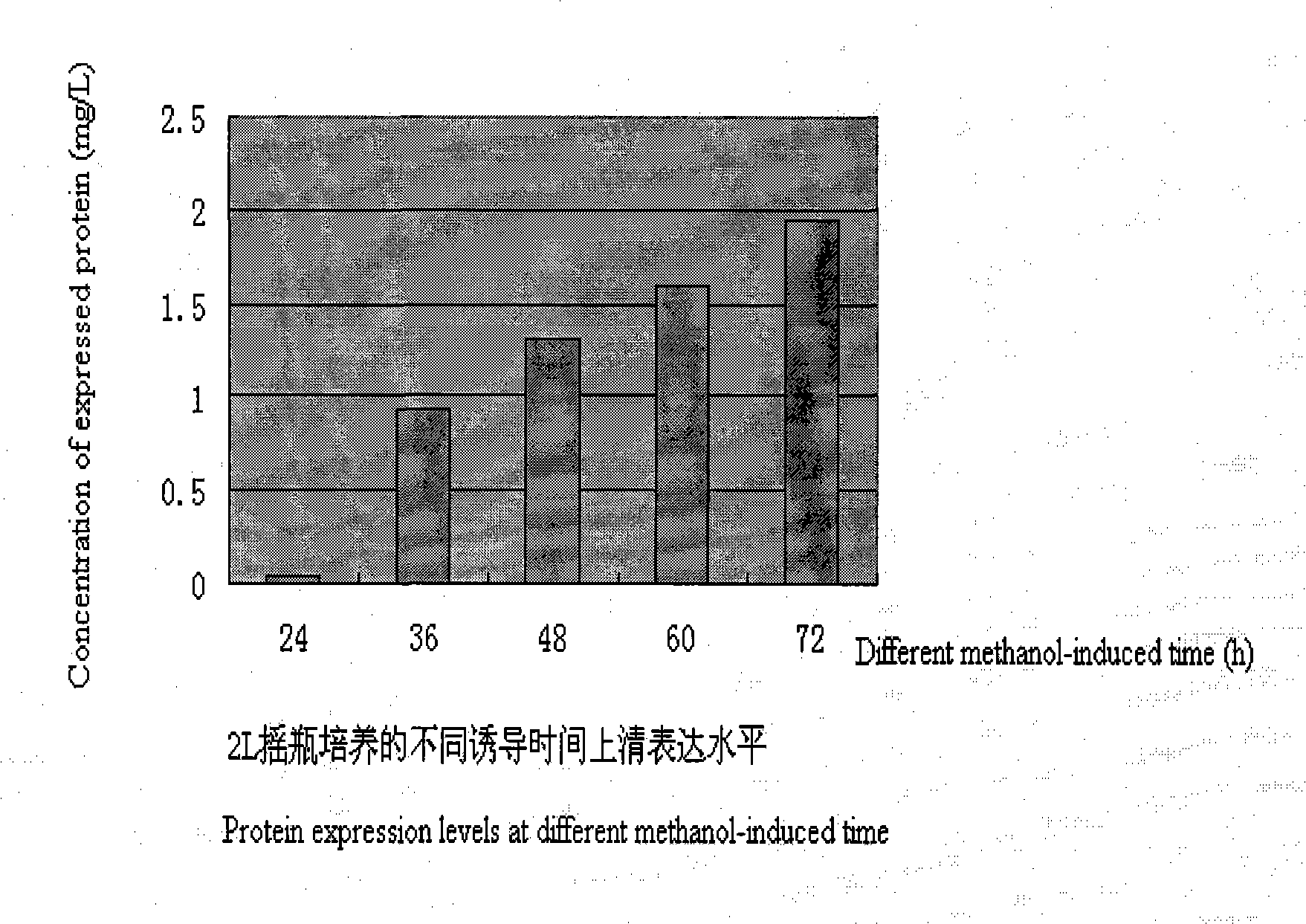
[0059] The pPIC9-sTNFRII-IgGFc2 plasmid constructed in Example 2 was linearized with Bgl II, and the prepared α-1,6-mannosyltransferase (Ochlp) gene knockout Pichia pastoris GS115 host bacteria was electrotransformed, and its Spread onto MD flat panels. Cultured at 30°C for 3 days to obtain yeast transformants. The transformants were inoculated into YPD medium, and cultured at 250 rpm for 24 hours at 30°C. Inoculate 25mL BMGY medium with 4% inoculum in 30°C and 250rpm for 24h, then start 0.05% methanol adaptation induction, 12h after 0.5% methanol induction, and then add 0.5% me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com