Preparation and application of recombinant adenovirus vector bivalent living vaccine for herpes progenitalis
A recombinant adenovirus, herpes simplex virus technology, applied in the direction of virus/phage, application, antiviral agent, etc., can solve the problems of low response ability, carcinogenic risk, weak immunogenicity, etc., to control recurrence and transmission, high infection ability, the effect of reducing the incidence of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
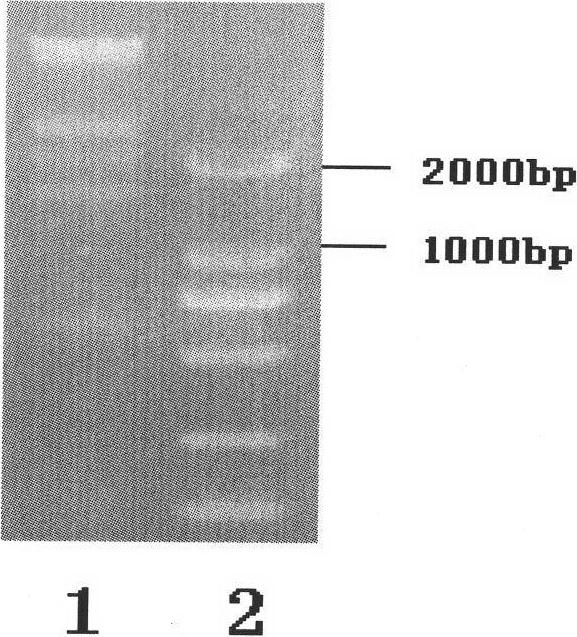
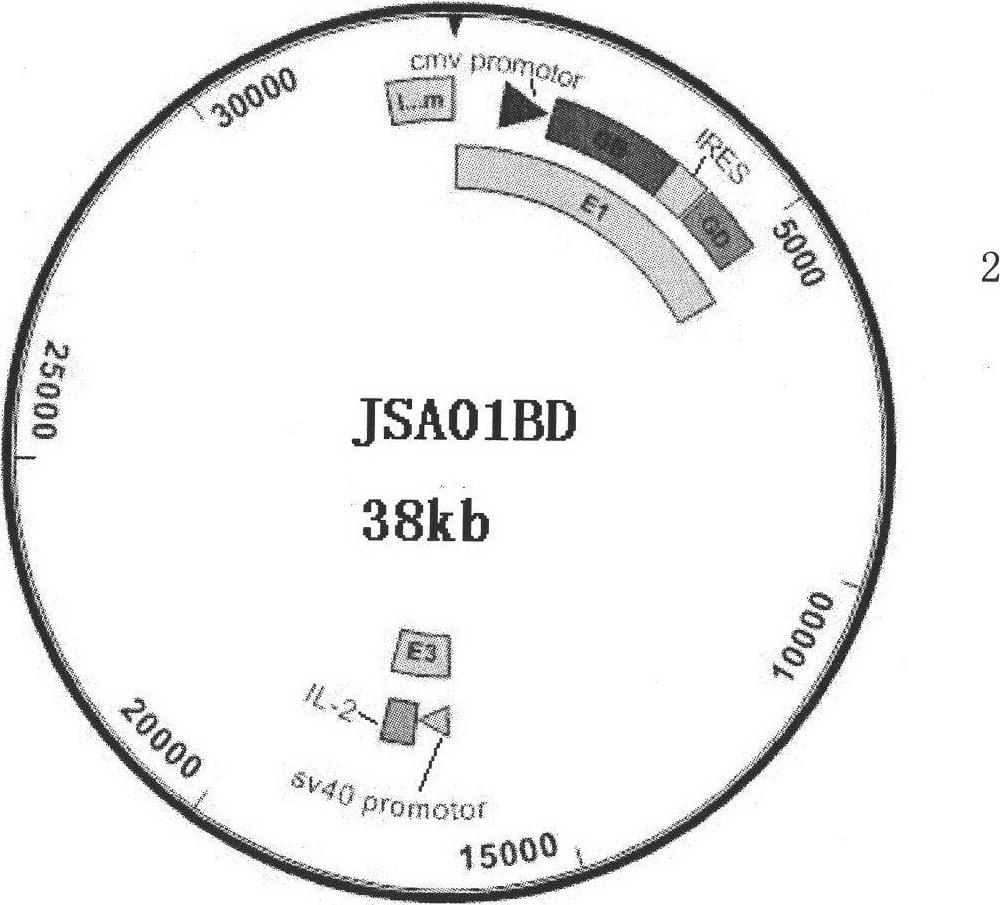
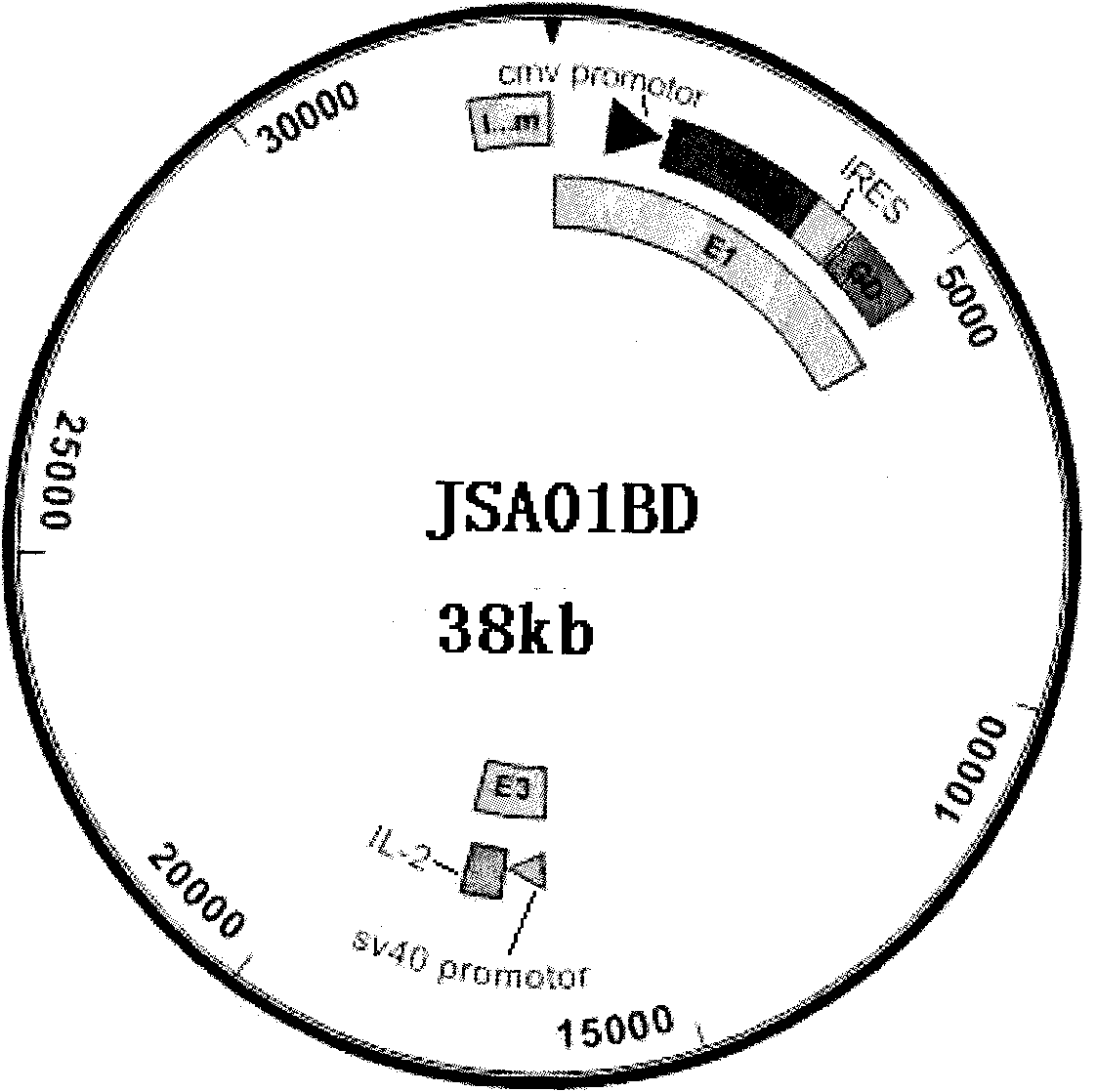
[0014] The preparation of embodiment 1 recombinant adenovirus vector bivalent live vaccine JSA01BD
[0015] 1. Isolation of wild-type HSV-2
[0016] (1). Take the herpes fluid and secretions of patients with clinical HSV infection, add 1ml of PBS buffer solution and filter with a 0.45 micron filter.
[0017] (2). Take a six-well plate, pass 2×10 per well 5 For each Vero cell, 200 microliters of virus filtrate was added to each well of 5 wells, and one well was reserved as a control.
[0018] (3). After 72 hours of incubation in a 37°C carbon dioxide incubator, it was observed that cytopathic changes (CPE) appeared in the wells.
[0019] (4). After the cells in the wells of the six-well plate have complete CPE, put the medium and the cells into the centrifuge tube together.
[0020] (5). Freeze and thaw the centrifuge tube three times at 37°C-minus 80°C, centrifuge at 5000 rpm for 10 minutes, transfer the supernatant into a 2ml EP tube, and put it in a minus 80°C refrigerato...
Embodiment 2
[0126] Embodiment 2 Live vaccine JSA01BD is to the immune protection experiment of guinea pig genital herpes
[0127] 1. Experimental materials: wild-type herpes simplex virus type II: the titer was measured after amplifying HSV-2 with Vero cells, and the titer was about 1×107IU / ml; the titer of recombinant adenovirus padred and JSA01BD was 2×109IU respectively / ml and 1×10 9 IU / ml; 30 common-grade guinea pigs, female, weighing 275±25g, each of which is marked on different parts of its body, and the corresponding marks are converted into 10 Arabic numerals from 1 to 30, according to random According to the chemical principle, 10 rats / group were divided into three groups: blank control group, negative control group and positive group.
[0128] 2. Immunization and attack plan
[0129] Immunity dose: 10 8 TCID50 recombinant adenovirus; route: intranasal instillation, primary immunization at week 0, booster immunization after 2 weeks (d14), and challenge with wild-type HSV-2 af...
Embodiment 3
[0153] Embodiment 3 Live vaccine JSA01BD is to the immune protection experiment of mouse
[0154] 1. Experimental materials: wild-type herpes simplex virus type II: the titer was measured after amplifying HSV-2 with Vero cells, and the titer was about 1×107IU / ml; the titer of recombinant adenovirus padred and JSA01BD was 2×109IU respectively / ml and 1×10 9 IU / ml; ordinary level experimental mice, 30, female, weighing 20±2g, each of which is marked on different parts of its body, after converting the corresponding marks into 10 Arabic numerals from 1 to 30, press According to the principle of randomization, 10 rats / group were divided into three groups: blank control group, negative control group and positive group.
[0155] 2. Immunization and attack plan
[0156] Immunity dose: 10 8 TCID50 recombinant adenovirus; route: intranasal instillation, primary immunization at week 0, booster immunization after 2 weeks (d14), and challenge with wild-type HSV-2 after another two week...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com