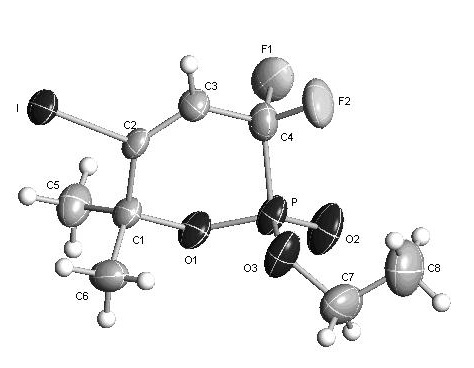Method for synthesizing alpha-difluoro methylene phosphonic lactone
A technology of difluoromethylenephosphonolactone and difluoromethylene, which is applied in the field of synthesis of α-difluoromethylenephosphonolactone, can solve the problem of lack of synthesis methods, The biological activity of esters has not been found in the open literature and other issues, and the method is simple, the yield is high, and the conditions are mild.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] in N 2 Under gas protection, add 0.5 mmol 4,4-dimethyl-1,1-difluoromethylene-2,3-alkenylphosphonic acid monoethyl ester, 0.75 mmol NIS and 6 mL CH 3 CN, normal temperature reaction, TLC tracking, dilute with ethyl acetate after the reaction, followed by 5% Na 2 S 2 o3 The solution was washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and the solvent was removed by rotary evaporation. The crude product was purified by flash column chromatography.
[0037] The structure of the obtained phosphonolactone is as follows, and the yield is 88%.
[0038]
[0039] m.p.: 90-91 °C.
[0040] IR (KBr): 1623, 1268, 1046, 1005 cm -1 .
[0041] 1 H NMR (300 MHz, CDCl 3 ): δ 6.56-6.45 (m, 1 H), 4.36-4.26 (m, 2 H), 1.75 (s, 3 H), 1.69 (s, 3 H), 1.36 (t, J = 6.9 Hz, 3 H).
[0042] 13 C NMR (100 MHz, CDCl 3 ): δ 132.6-131.9 (m), 116.3-116.1(m), 108.6 (td, J C-F = 254.0 Hz, J C-P = 200.0 Hz), 89.4 (d, J C-P = 8.0 Hz), 65.6 (d, J C-P = 6.0 ...
Embodiment 2
[0049] in N 2 Under air protection, add 0.5 mmol 4-(1,4-butylene)-1,1-difluoromethylene-2,3-alkenylphosphonic acid monoethyl ester, 0.75 mmol NIS and 6 mL CH 3 CN, normal temperature reaction, TLC tracking, dilute with ethyl acetate after the reaction, followed by 5% Na 2 S 2 o 3 The solution was washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and the solvent was removed by rotary evaporation. The crude product was purified by flash column chromatography.
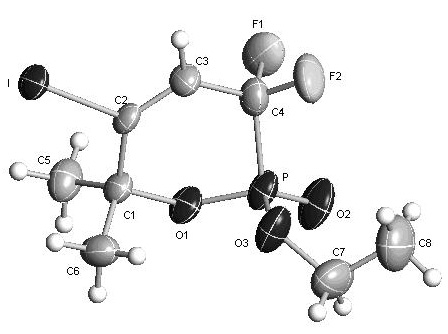
[0050] Its structure of the obtained phosphonolactone is as follows,
[0051]
[0052] m.p.: 101-102 °C.
[0053] IR (KBr): 1619, 1287, 1044, 1000 cm -1 .
[0054] 1 H NMR (300 MHz, CDCl 3 ): δ 6.69-6.57 (m, 1 H), 4.41-4.31 (m, 2 H), 2.40-2.33 (m, 3 H), 2.19-2.11 (m, 1 H), 2.00-1.72 (m, 4 H), 1.42 (t, J = 6.9 Hz, 3 H).
[0055] 13 C NMR (125 MHz, CDCl 3 ): δ 133.9-133.4 (m), 116.4-116.2 (m), 109.5 (td, J C-F = 255.2 Hz, J C-P = 200.5 Hz), 98.9 (d, J C-P = 8.8 Hz), 65.7 ...
Embodiment 3
[0062] in N 2 Under gas protection, add 0.5 mmol 4,4-diethyl-1,1-difluoromethylene-2,3-alkenylphosphonic acid monoethyl ester, 1 mmol I 2 and 6 mL CH 3 CN, normal temperature reaction, TLC tracking, dilute with ethyl acetate after the reaction, followed by 5% Na 2 S 2 o 3 The solution was washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and the solvent was removed by rotary evaporation. The crude product was purified by flash column chromatography.
[0063] Its structure of the obtained phosphonolactone is as follows,
[0064]
[0065] m.p.: 39-40 °C.
[0066] IR (KBr): 1619, 1285, 1053, 1001 cm -1 .
[0067] 1 H NMR (300 MHz, CDCl 3 ): δ 6.76-6.64 (m, 1 H), 4.39-4.31(m, 2 H), 2.11-1.94 (m, 4 H), 1.41 (t, J = 6.9 Hz, 3 H), 1.02 (t, J =7.2 Hz, 3 H), 0.96 (t, J =7.2 Hz, 3 H).
[0068] 13 C NMR (CDCl 3 , 125 MHz): δ 134.6-134.1 (m), 115.2-115.0 (m), 110.0 (td, J C-F = 253.7 Hz, J C-P = 199.1 Hz), 94.5 (d, J C-P = 9.8 Hz), 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com