Deuterated bensulfuron-methyl and intermediate 2-amino-4,6-dideutero methoxypyridine and preparation method thereof
A technology for substituted methoxypyrimidine and bensulfuron-methyl compound, which is applied in the field of deuterated bensulfuron-methyl and intermediate 2-amino-4,6-dideuterated methoxypyrimidine and preparation, and can solve difficult problems. Control the quality of deuterated compounds, the inability to separate deuterium-substituted compounds and hydrogen-substituted compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
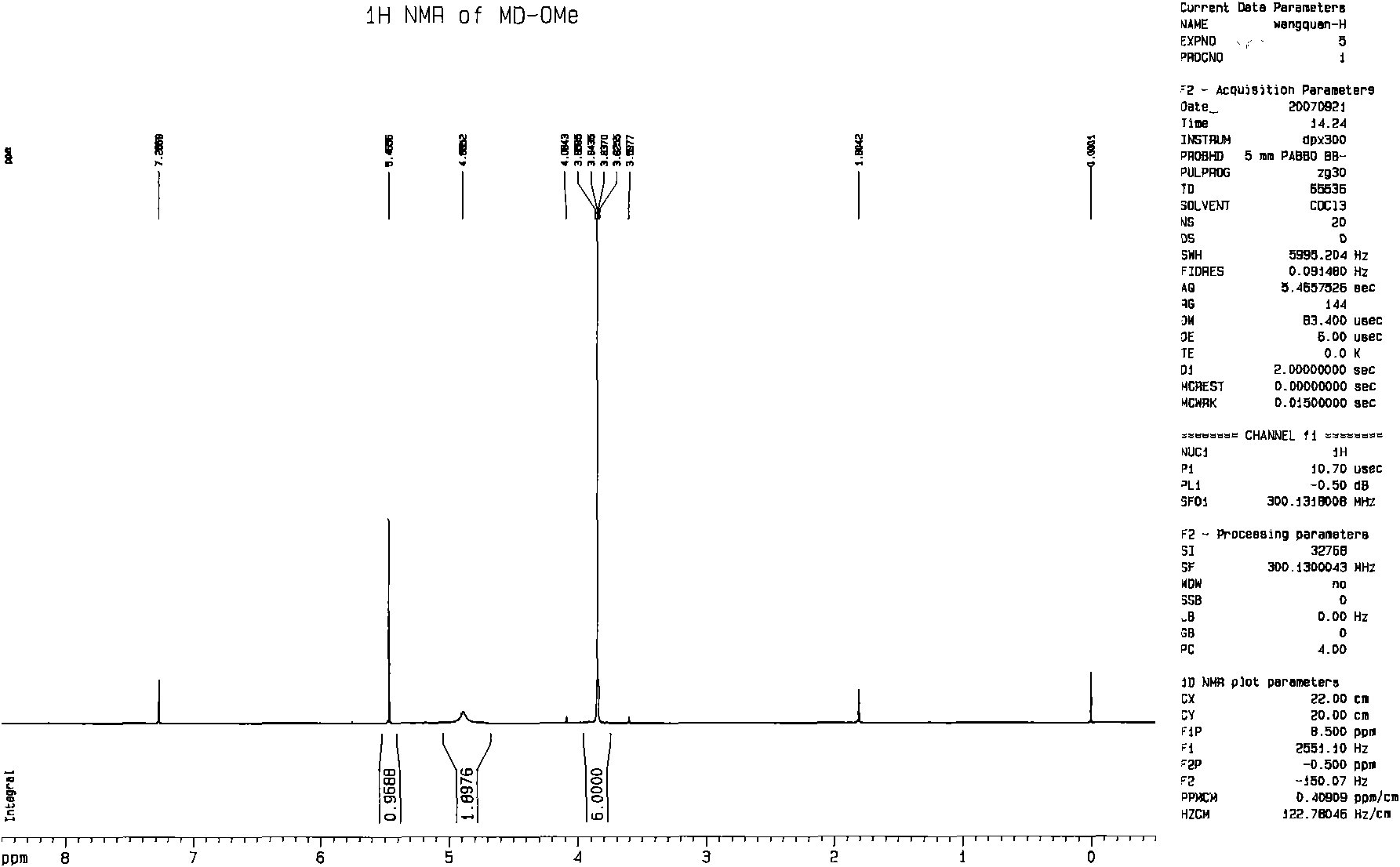
[0070] Example 1: Preparation of deuterated core unit 2-amino-4,6-dideuteriomethoxypyrimidine
[0071] Add 1g of sodium metal into a 50ml three-necked flask equipped with a stirring and condensing tube, then place 10ml of deuterated methanol in a constant pressure dropping funnel, and slowly drop it into the bottle depending on the boiling condition. After the sodium metal is completely reacted, 2 g of refined 2-amino-4,6-dichloropyrimidine is added, heated to 70° C. and refluxed for 6 hours. After the reaction, it was naturally cooled to room temperature, filtered, and then the filtrate was concentrated under reduced pressure, methanol was distilled off, and 6ml of water was added to precipitate a white solid. The resulting product is filtered and washed with water, dried, and weighed to obtain 1.25 g of the product
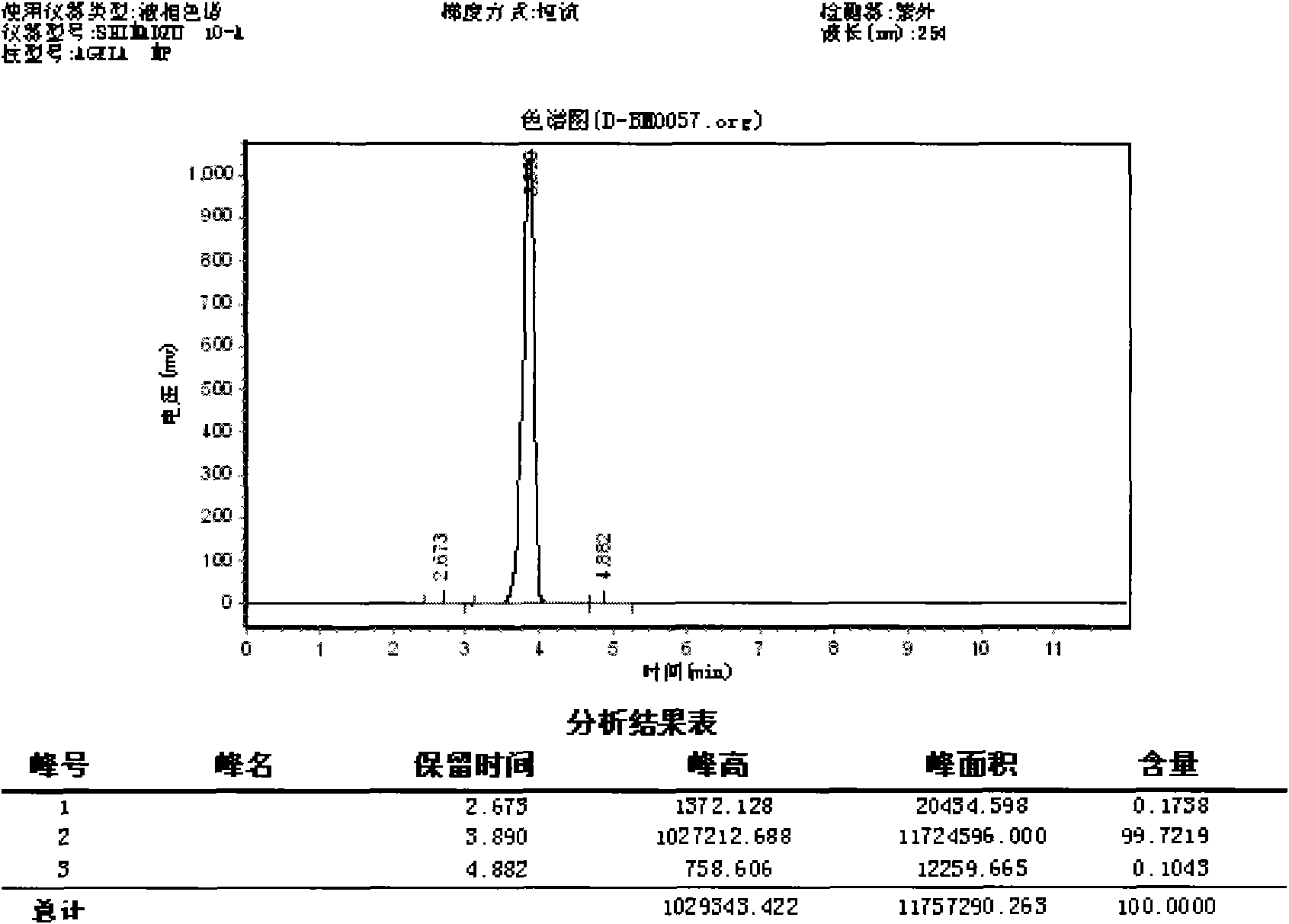
[0072] Deuterated core unit purity evaluation method:
[0073] The purity of 2-amino-4,6-dideuteriomethoxypyrimidine prepared in this experiment was analyzed ...
Embodiment 2
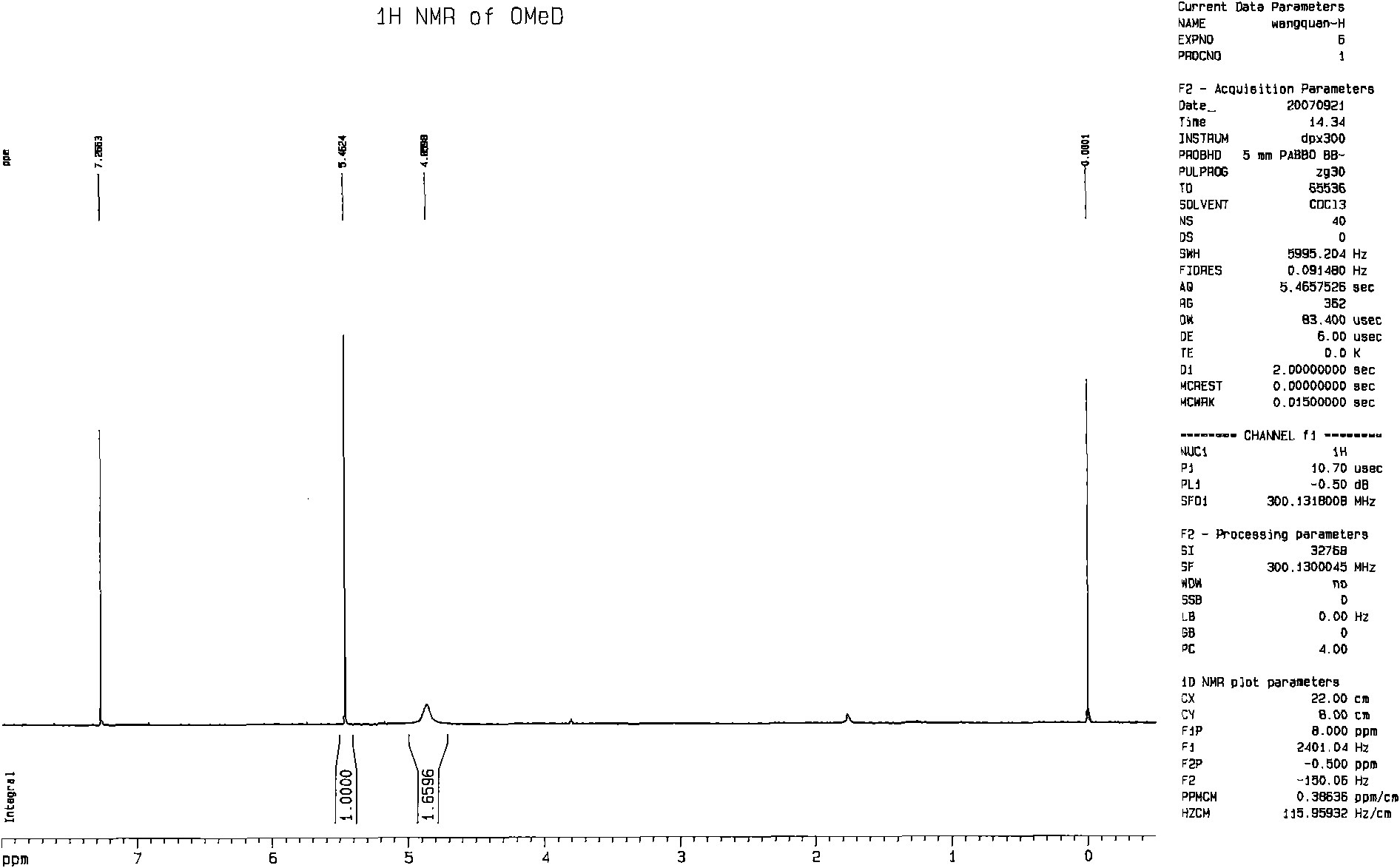
[0076] Embodiment 2: Preparation of deuterated bensulfuron-methyl
[0077] Add 1.15 g of o-ethoxyformyl benzylsulfonamide, 0.02 g of hexamethylenediamine and 10 ml of re-distilled dry xylene into a 50 ml three-neck flask equipped with a stirring and condensing tube, and then 0.54 g of solid trimerized Gas-dissolved in 6ml of re-evaporated and dried xylene and placed in a constant-pressure dropping funnel. The entire system needs to be shielded from light and air and protected with nitrogen, and a drying device and an exhaust gas absorption system should be installed. Heat the reaction bottle with an oil bath until the temperature of the reaction material is 90°C, start to slowly add the xylene solution of trimeric phosgene dropwise, take about three hours to complete the dropwise addition, and then reflux for another 2 hours.
[0078] Cool down to room temperature naturally after the reaction, filter, then concentrate the filtrate under reduced pressure, distill off most of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com