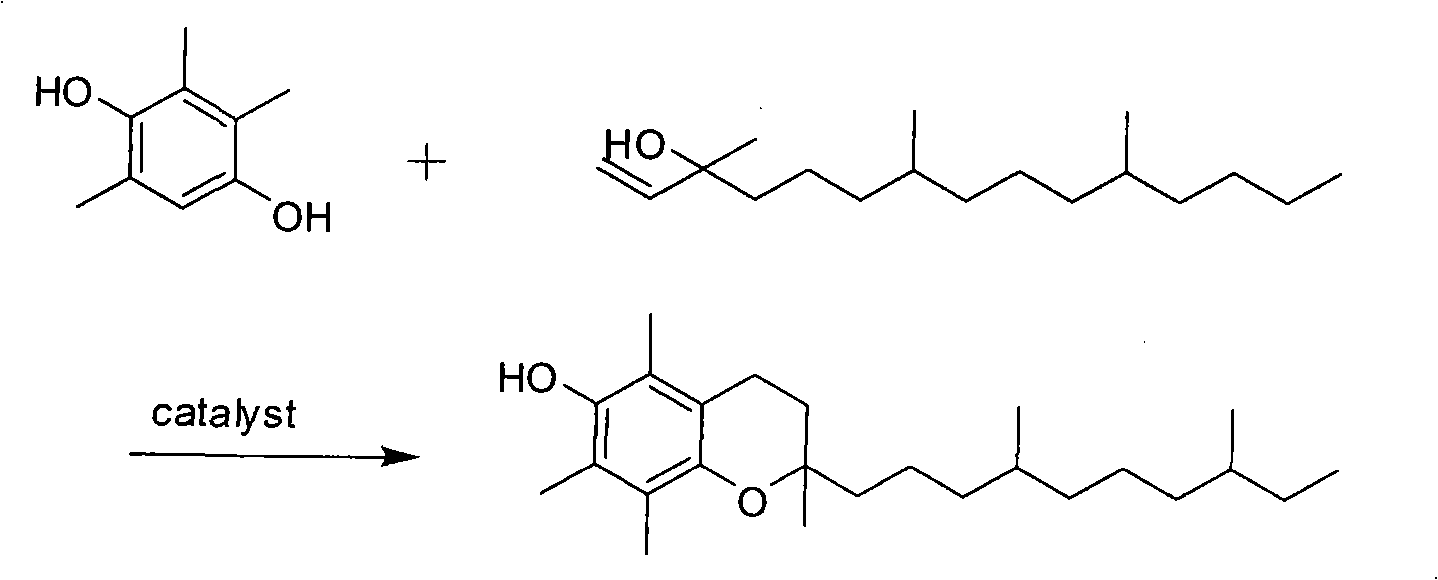
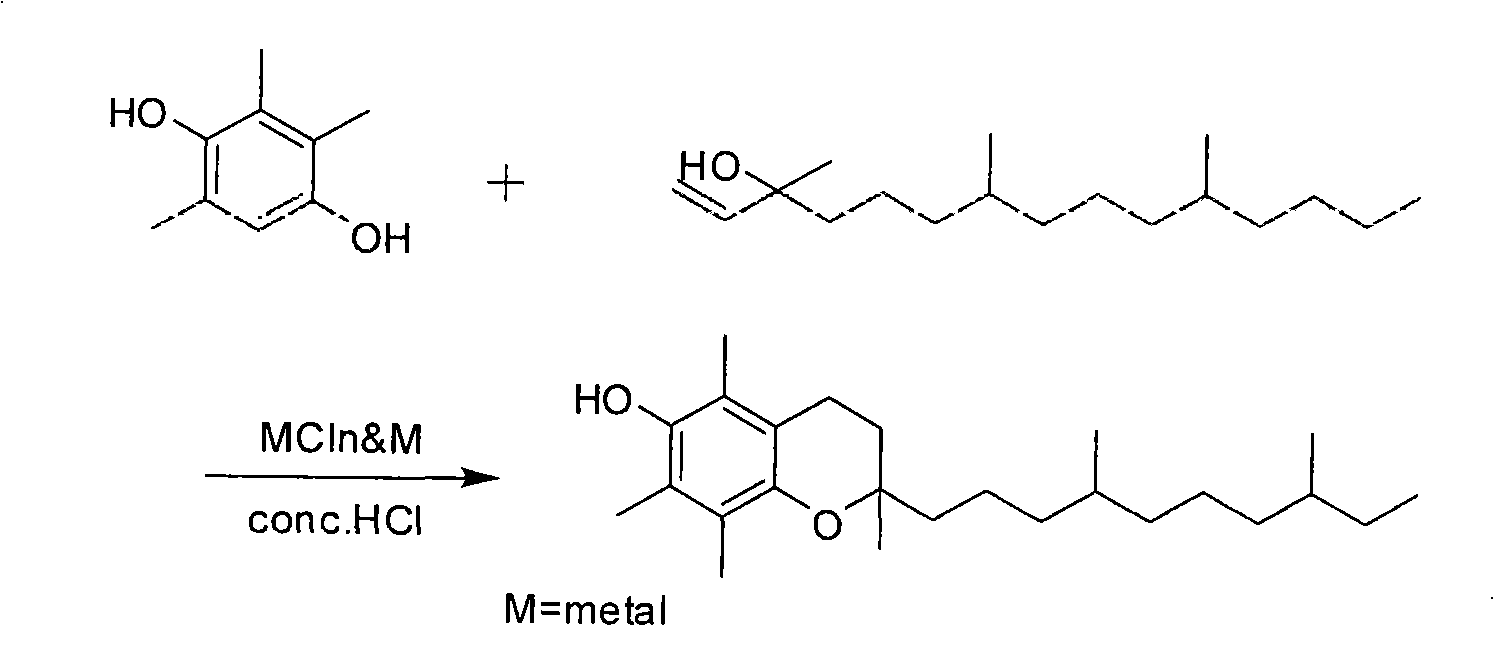
Preparation method of racemic tocopherol
A technology of tocopherol and trimethylhydroquinone, which is applied in the field of d, can solve the problems of expensive Lewis acid, deterioration, and increased procedures, and achieve the effect of easy-to-obtain raw materials, simple operation, and inhibition of oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Preparation of racemic tocopherol
[0021] Add 500 mL of ethyl acetate into a stirring 2L three-necked flask equipped with a condenser, start stirring, add 136 grams (1.0 mol) of zinc chloride, and after stirring evenly, add 152 grams of 2,3,5-trimethylhydroquinone (1.0mol), then slowly add 15mL of concentrated hydrochloric acid dropwise, after the raw material is dissolved, start to drop 300mL (1.1mol) of isophytic alcohol, control the temperature at 40°C, react for 5 hours, add 6.5g (0.1mol) of zinc powder , slowly warming up to 60°C for 2.5 hours. After the reaction, ethyl acetate was recovered under reduced pressure, the concentrate was dissolved in 500 mL of petroleum ether, 500 mL of distilled water was added to stand for liquid separation, and the solvent was recovered after drying to obtain 411 g of crude racemic tocopherol, with a yield of 110%.
Embodiment 2
[0022] Example 2 Preparation of racemic tocopherol
[0023] Add 500 mL of ethyl acetate into a stirring 2L three-necked flask equipped with a condenser, start stirring, add 88 grams (0.67 mol) of aluminum chloride, and add 152 grams of 2,3,5-trimethylhydroquinone after stirring evenly (1.0mol), then slowly add 15mL of concentrated hydrochloric acid dropwise, after the raw materials are dissolved, start to drop 270mL (1.0mol) of isophytic alcohol, control the temperature at 40°C, react for 6 hours, add 5.4g (0.2mol) of aluminum chips , slowly warming up to 60°C for 3 hours. After the reaction, ethyl acetate was recovered under reduced pressure, the concentrate was dissolved in 500 mL of petroleum ether, 500 mL of distilled water was added to stand for liquid separation, and the solvent was recovered after drying to obtain 430 g of crude racemic tocopherol, with a yield of 115%.
Embodiment 3
[0024] Example 3 Preparation of racemic tocopherol
[0025] Add 500 mL of ethyl acetate into a stirring 2L three-necked flask equipped with a condenser, start stirring, add 283 grams (1.5 mol) of tin chloride, and after stirring evenly, add 152 grams of 2,3,5-trimethylhydroquinone (1.0mol), then slowly add 15mL of concentrated hydrochloric acid dropwise, after the raw material is dissolved, start to add 400mL (1.5mol) of isophytol dropwise, control the temperature at 40°C, react for 6 hours, add 6g (0.05mol) of tin powder , slowly warming up to 60°C for 3.5 hours. After the reaction, ethyl acetate was recovered under reduced pressure, the concentrate was dissolved in 500 mL of petroleum ether, 500 mL of distilled water was added to stand for liquid separation, and the solvent was recovered after drying to obtain 400 g of crude racemic tocopherol with a yield of 107%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com