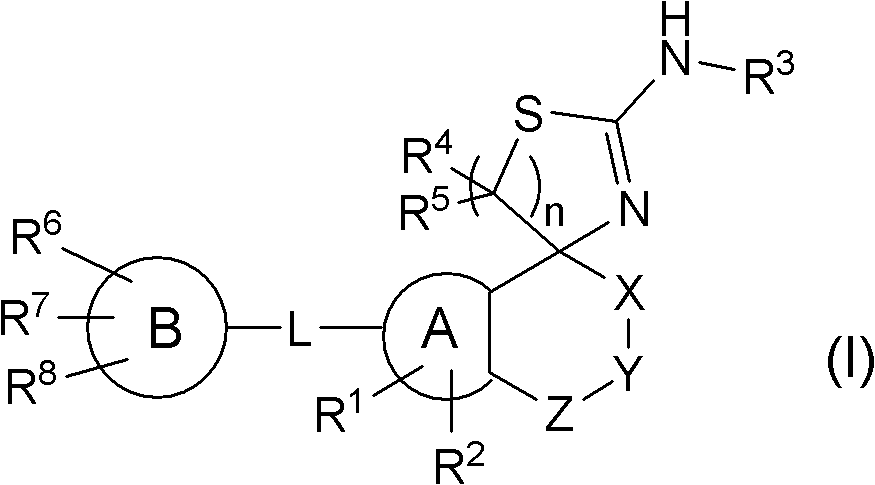
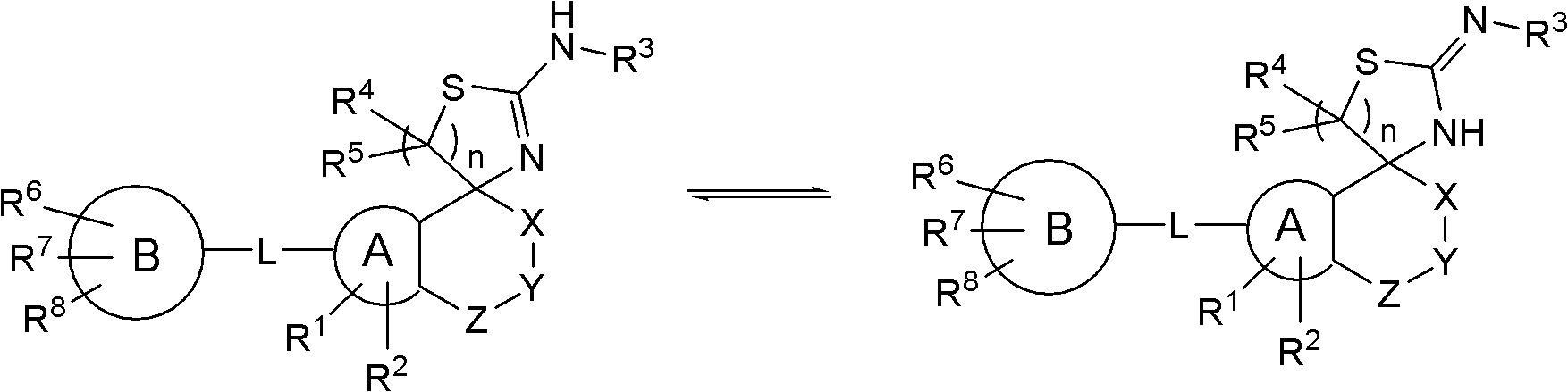
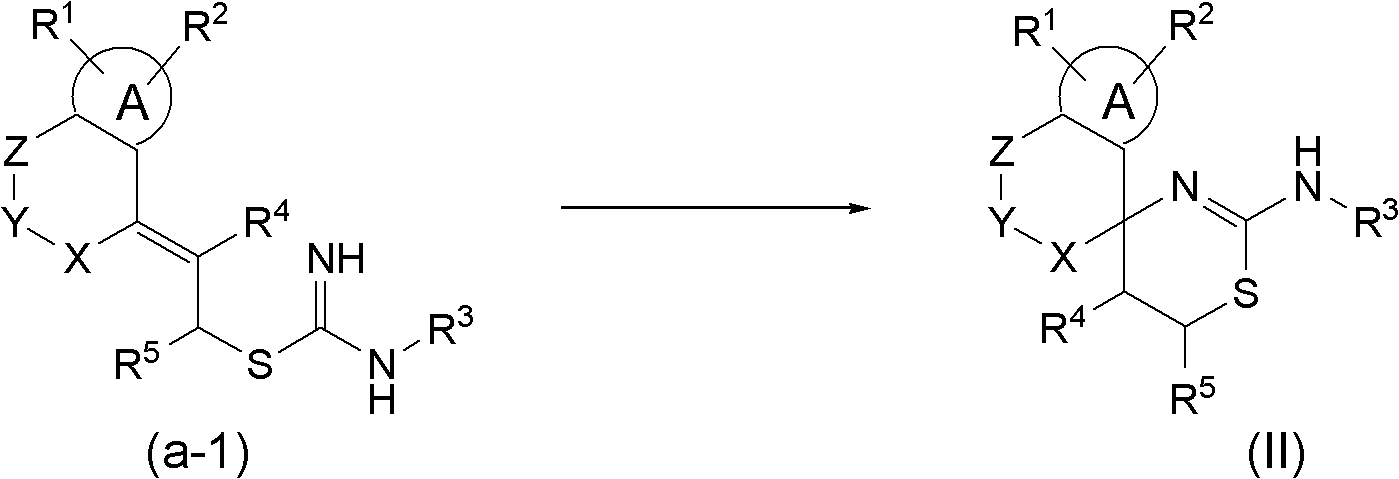
Spiroaminodihydrothiazine derivatives
An amino and group technology, applied in the field of spiroamino dihydrothiazine derivatives and their pharmaceutical applications, can solve the problem that basic drug therapy has not yet been developed and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0261] Synthesis of 8-Fluorochroman-4-one (Compound 1-3)
[0262] [Formula 8]
[0263]
[0264] (1) Synthesis of 3-(2-fluorophenoxy)propionic acid (compound 1-2)
[0265] (1-1) A solution of 2-fluorophenol (compound 1-1, 3.0g) in N,N-dimethylformamide (7.0ml) was added to sodium hydride (3.22 g) in a 50% solution in N,N-dimethylformamide (100ml). After stirring at the same temperature for 30 minutes, a solution of 3-bromo-propionic acid (4.91 g) in N,N-dimethylformamide (8.0 ml) was added. The mixture was returned to room temperature and stirred at the same temperature for 24 hours. The reaction was terminated by adjusting the pH to 1-2 with 1N hydrochloric acid (100ml). The aqueous layer was extracted with ethyl acetate, and the organic layer was washed with brine. The organic layer was dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was crystallized by adding a 20% ethyl acetate-hexane mixed solvent to o...
preparation example 2
[0276] Synthesis of 2,3,5',6'-tetrahydrospiro[benzopyran-4,4'-[1,3]thiazin]-2'-amine (compound 1)
[0277] [Formula 9]
[0278]
[0279] (1) Synthesis of 4-vinyl-chroman-4-ol (compound 2-2)
[0280] Zinc chloride (461 mg) was added to vinylmagnesium chloride (1.48 M solution in tetrahydrofuran; 29.7 ml), and the mixture was stirred at room temperature for 1 hr. The reaction solution was cooled to 0°C, followed by dropwise addition of a solution (20.0 ml) of compound 2-1 (5.00 g) in tetrahydrofuran. The reaction solution was stirred at the same temperature for 5 hours. After confirming the disappearance of the starting material, ammonium chloride solution was added to the reaction mixture. The aqueous layer was extracted with ethyl acetate, and the organic layer was washed with brine. The organic layer was dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The obtained crude product was purified by silica gel column chroma...
preparation example 3
[0289] Synthesis of N-(2'-amino(6-nitro-2,3,5',6'-tetrahydrospiro[benzopyran-4,4'-[1,3]thiazin]-6-yl ) Trifluoroacetamide (Compound 2)
[0290] [Formula 10]
[0291]
[0292] (1) Synthesis of 2,2,2-trifluoro-N-(4-oxochroman-6-yl)acetamide (compound 3-3)
[0293] Compound 3-1 (1.0 g) was dissolved in acetone (50 ml). Tin chloride dihydrate (3.66 g) was added and the mixture was heated at reflux overnight. After confirming that the reaction was complete, the reaction mixture was cooled to room temperature. The solvent was evaporated under reduced pressure, and aqueous sodium bicarbonate solution was added to the reaction mixture, followed by extraction with dichloromethane. The aqueous layer was back extracted with ethyl acetate. The organic layer was dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was purified by silica gel column chromatography. The obtained product (464 mg) was dissolved in dichloromet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com