Electrolytic gold plating solution and gold film obtained using same
A technology for electroplating gold and gold film, applied in the field of gold film, can solve problems such as difficulty, impracticality, consumption, etc., and achieve a good effect of gold precipitation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~10、 comparative example 1~12
[0086] With respect to all the gold plating solutions, 9 g / L of potassium gold cyanide was dissolved in terms of gold, and 200 ppm of cobalt salts, nickel salts, or iron described in each of the examples and comparative examples shown in Table 1 were dissolved in terms of metal. For the salt, dissolve 1000ppm of a specific heterocyclic compound or its comparative compound, dissolve 100g / L of citric acid as a component serving as both a conductive salt and a buffer, adjust the pH to 4.3, and prepare a gold plating solution.
[0087] As the "comparative compound", hexamethylenetetramine, a benzene ring compound in which one or more nitro groups were substituted on the carbon atoms in the ring, and a heterocyclic compound in which a nitro group was not substituted were used. In addition, the pH was adjusted with a 20% by mass potassium hydroxide aqueous solution and citric acid, the bath temperature of the gold plating solution was set at 50° C., and the following evaluations were...
Embodiment 11
[0089] Except not containing metal salts such as cobalt salts, nickel salts, iron salts, etc. except gold salts, prepare the electroplating gold solution in the same manner as in Example 1, implement electroplating in the same manner as in Example 1, and perform the following steps in the same manner. evaluate.
[0090]
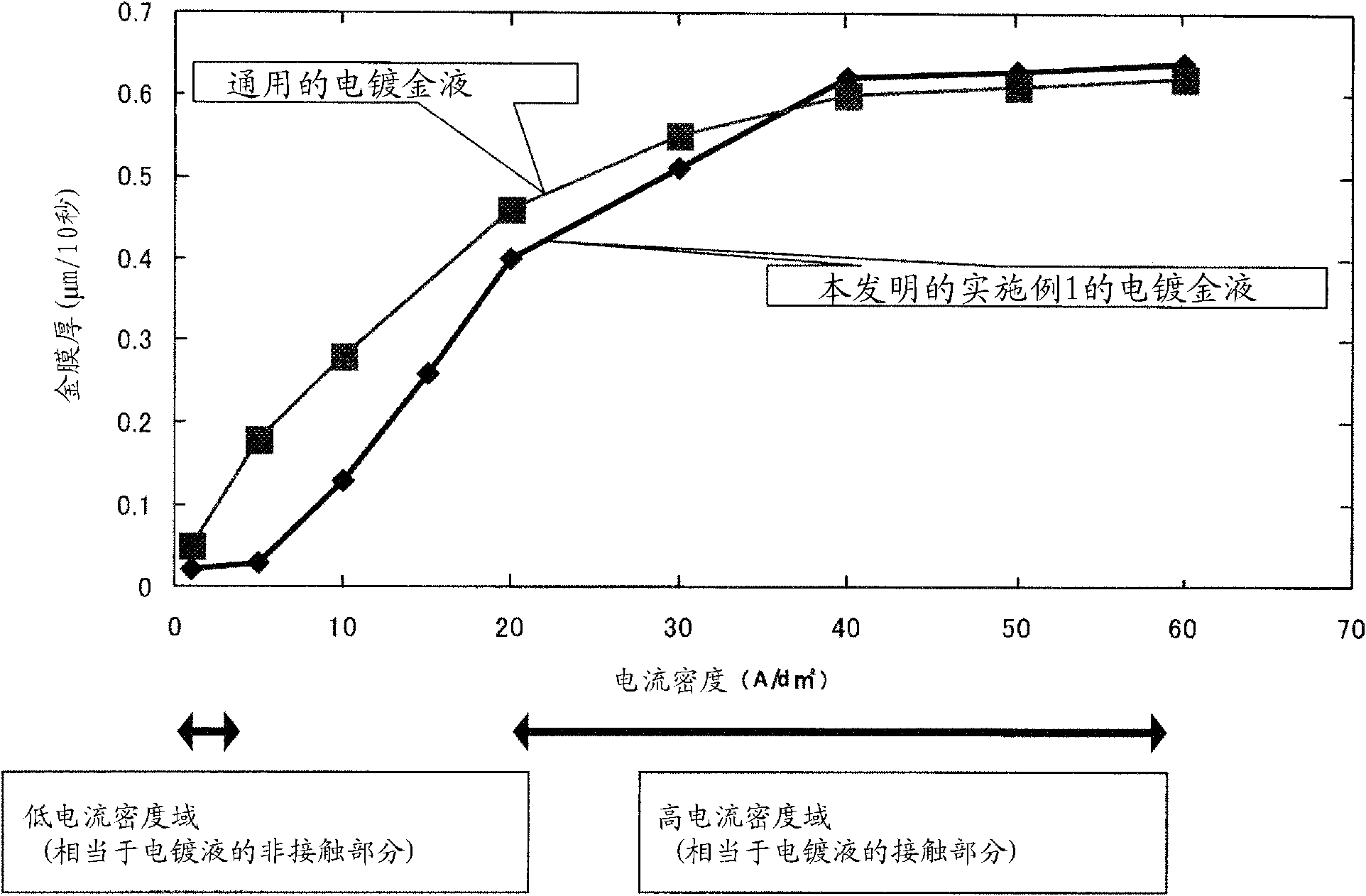
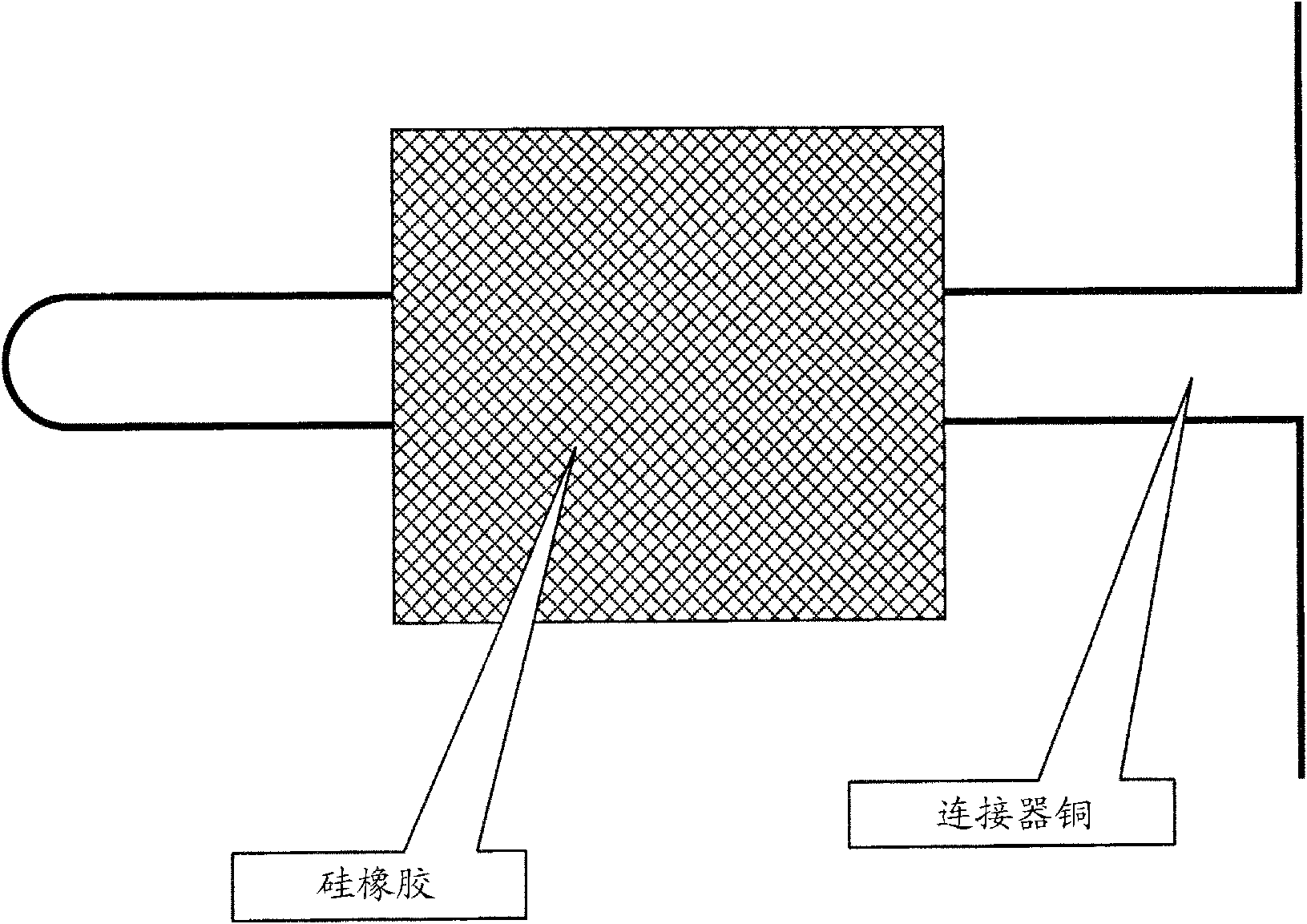
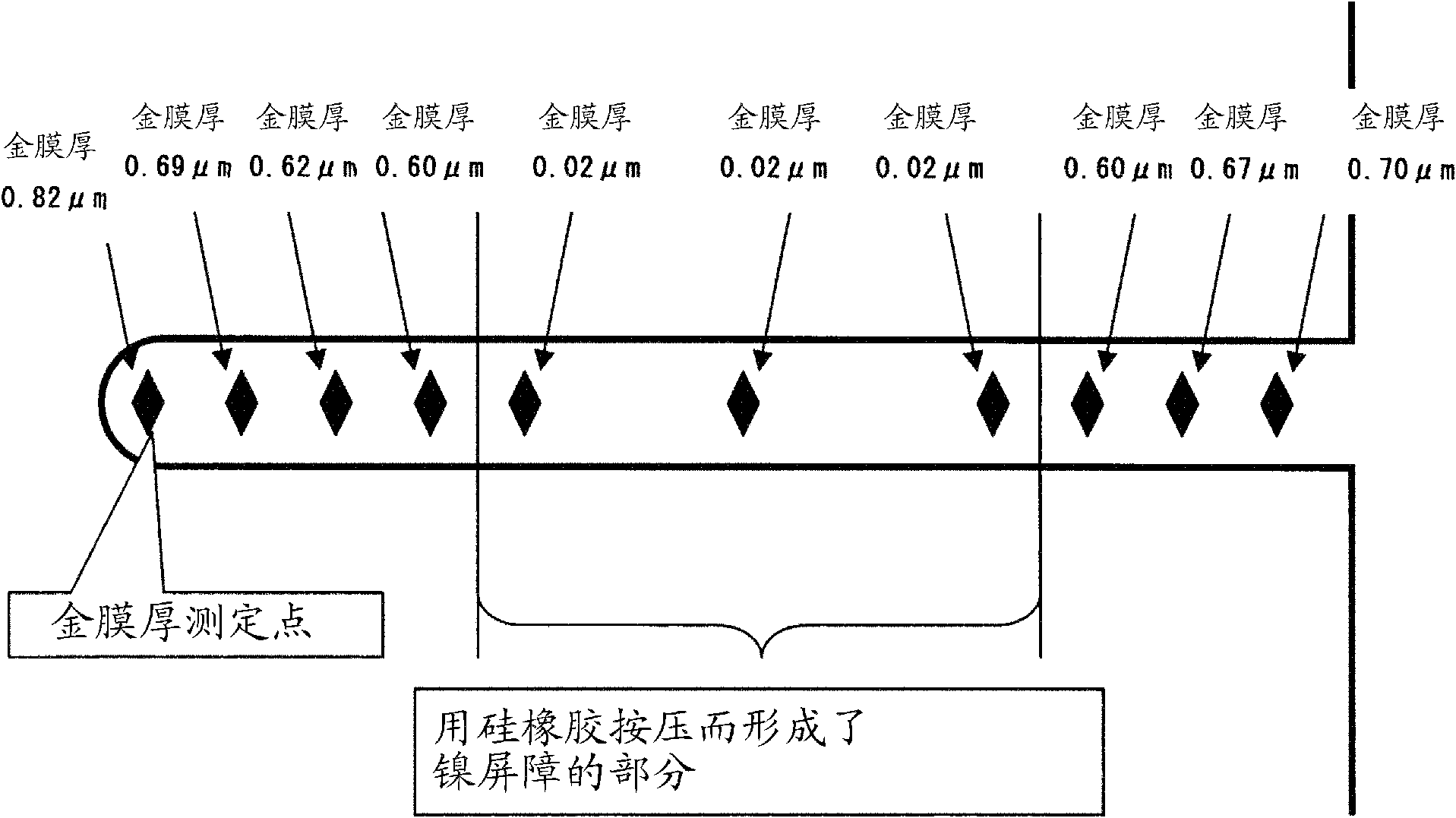
[0091] Using the gold plating solution prepared in each of the Examples and Comparative Examples, gold was electroplated on a 2.0 μm primary bright nickel plating film on a 10 mm×10 mm copper plate according to the procedure shown in Table 2. For electroplating gold, the electroplating solution is agitated by the pump jet flow (hereinafter referred to as "jet flow type gold plating method") at a flow rate of 18L per minute from a circular jet outlet with a diameter of 8mm, while the current density is 5A / dm 2 、40A / dm 2 The two levels were electroplated with gold for 10 seconds each.
[0092] In addition, for the primary bright nickel plating film, the f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com