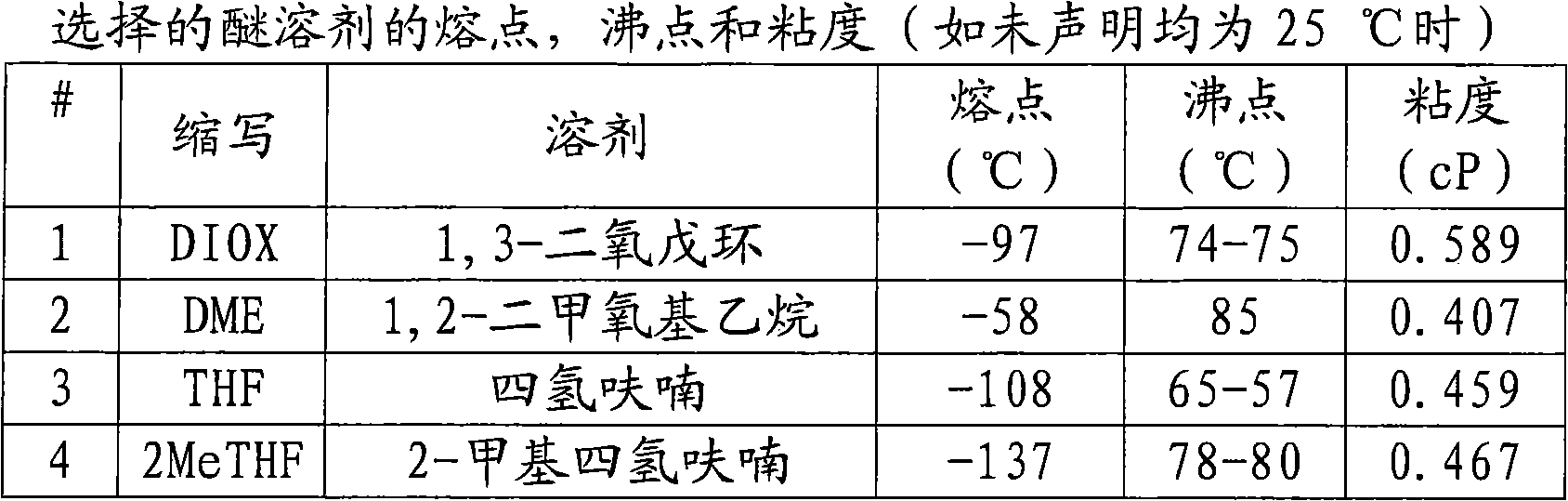
THF-based electrolyte for low temperature performance in primary lithium batteries
An electrolyte and battery technology, used in primary batteries, battery electrodes, large-sized batteries/batteries, etc., can solve problems such as low operating voltage, health and safety hazards, and voltage drop
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] according to figure 1 and 2 The information contained in constructs the battery. The cells were cooled from 21°C to 0°C, -20°C and -40°C. Cell impedance data were recorded by sweeping the frequency from 65 kHz to 1 Hz after 1 hour equilibration at each temperature.
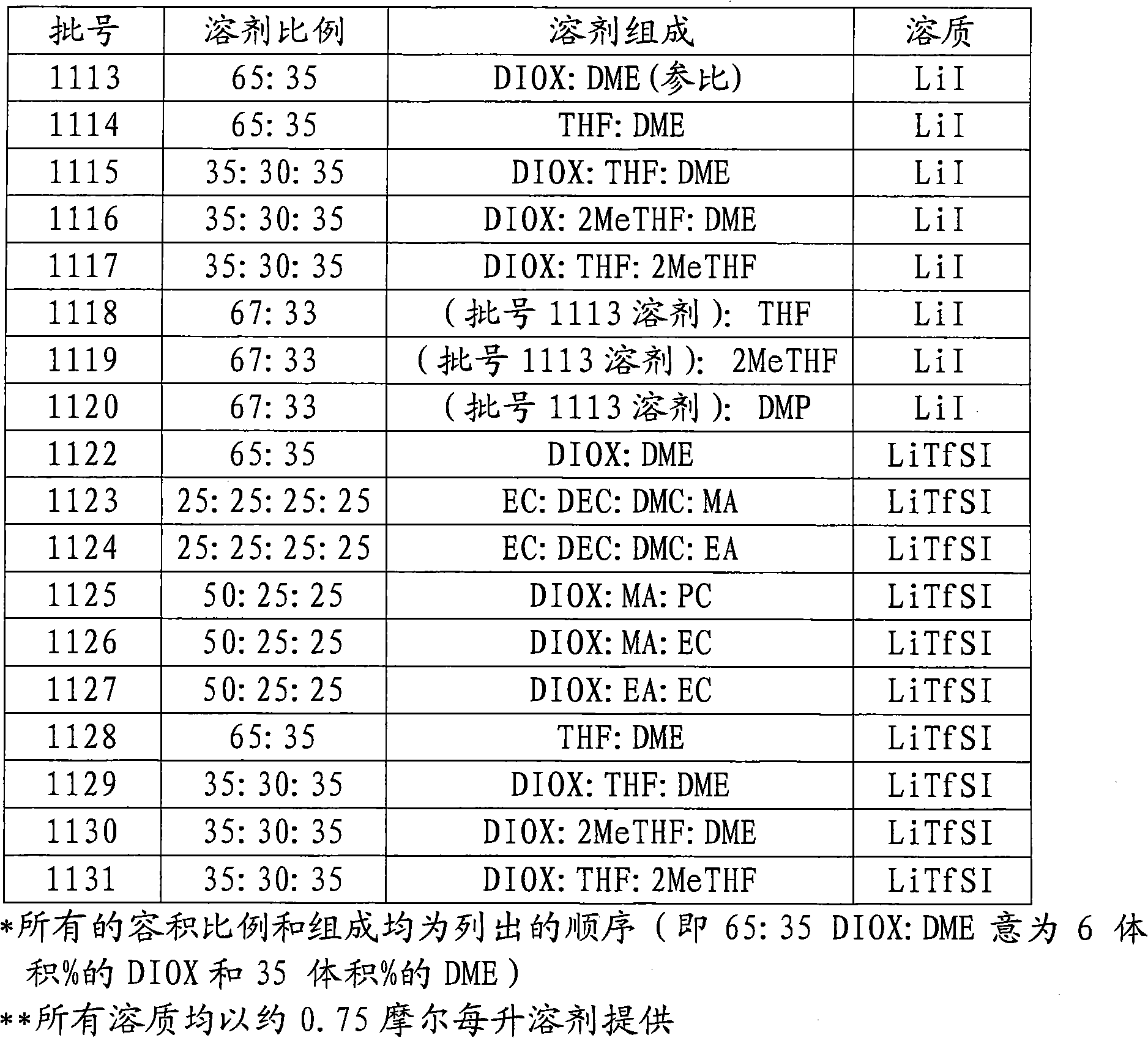
[0085] The addition of THF, 2MeTHF, and DMP slightly suppressed the OCV of the cells, while the addition of PC and EC to the DIOX-containing solvent significantly increased the OCV of the cells compared to the "reference" electrolyte containing only DIOX and DME. And the cells using lithium imide have a slightly higher OCV than the cells using LiI salt.
[0086] In terms of cell impedance, the reference batch using a 65:35 DIOX:DME (all ratios mentioned here are volume ratios mixed at room temperature) solvent mixture had the lowest impedance when using the LiI salt. THF and 2MeTHF slightly increased the impedance when the solvent mixture contained both DIOX and DME. However, THF-DME and DIOX-THF-2MeT...
Embodiment 2
[0088]The signature test was performed as follows: The cells in Example 1 (including the cooling rules described here) were continuously discharged at a gradually decreasing drain rate, after reaching the cut-off voltage (both 1.0 and 0.9 volts for ease of comparison). A normalized stop time after a volt cut. The battery is then tested at the next drain rate, and the test continues until completion. However, for the initial 1A discharge, the current is briefly interrupted for 100mS every minute of discharge, during which the resistance of the battery can be observed. For all low temperature tests, the test schedule also included a 5 hour delay to achieve a minimum dwell time of 2-3 hours at the specified test temperature.
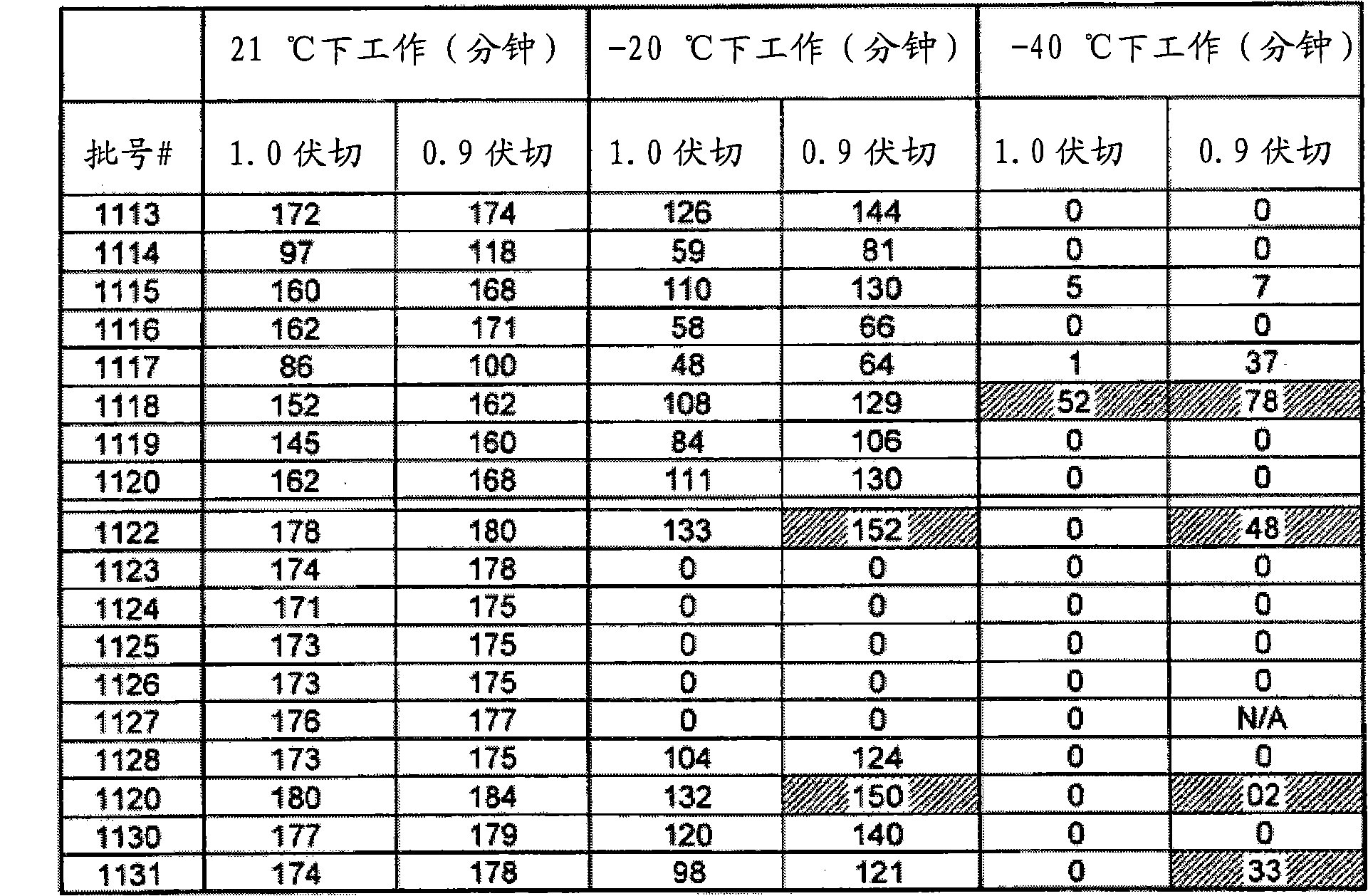
[0089] image 3 The work in phase 1A of the signature test at three different temperatures (21°C, -20°C and -40°C) is summarized. When the salt was changed from LiI to lithium imide, they performed the same or only slightly worse than the reference solve...
Embodiment 3
[0095] Further tests were performed with the cells of Example 1 (including the cooling rules described here), but this time with the optimal composition of DIOX:DME:THF with the best low temperature (-40°C) and ambient temperature performance LiI electrolyte of solvent mixture.
[0096] Compared to previous teachings (such as references in JES, 131, 2821 by Matsuda et al.), the cell in this example shows unexpected advantages at -40°C.
[0097] Design by Stat-Ease, Inc. Version 7.1.3 optimizes the solvent composition. Therefore, the following optimized solvent composition focusing on improving the low-temperature performance of the battery is 57.3 wt% DIOX, 23.8 wt% DME and 18.9 wt% THF.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com