Method for producing hydrochloric acid and silicon dioxide by using silicon tetrachloride as raw material
A technology of silicon dioxide and silicon tetrachloride, applied in the direction of silicon dioxide, silicon oxide, chlorine/hydrogen chloride, etc., can solve the problems of low utilization value, high production cost, complicated process, etc., and achieve good promotion and application prospects, The effect of low equipment requirements and simple reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In this embodiment, the method for producing hydrochloric acid co-production silica with ceramic balls as the reaction carrier, the specific steps are as follows:
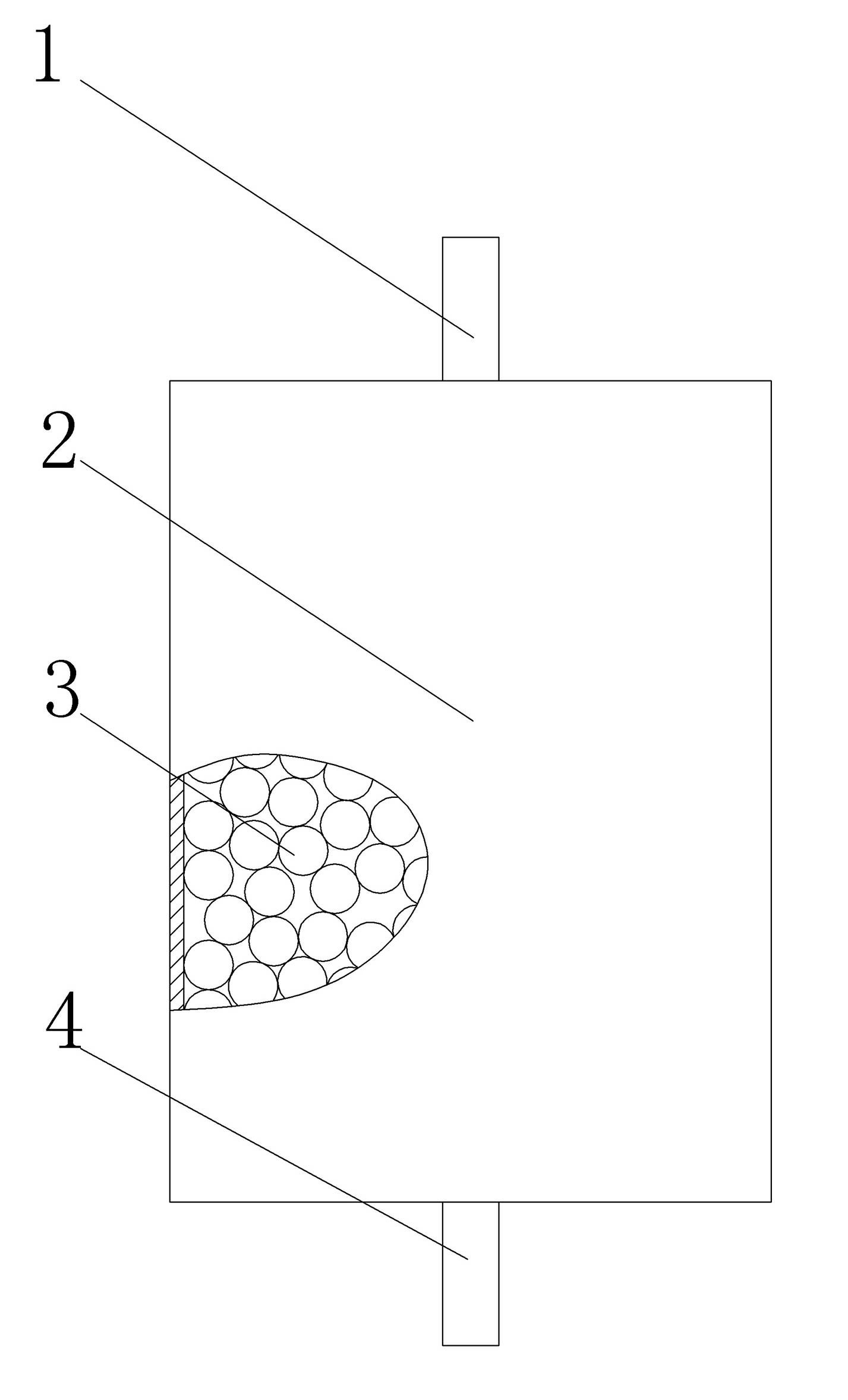
[0022] (1) if figure 1 As shown, in the reaction vessel 2, ceramic balls 3 with a diameter of about 10mm are piled up, and the quality of the accumulation is 20kg, and the bottom and top of the reaction vessel are respectively provided with inlet and outlet ports 4,1;
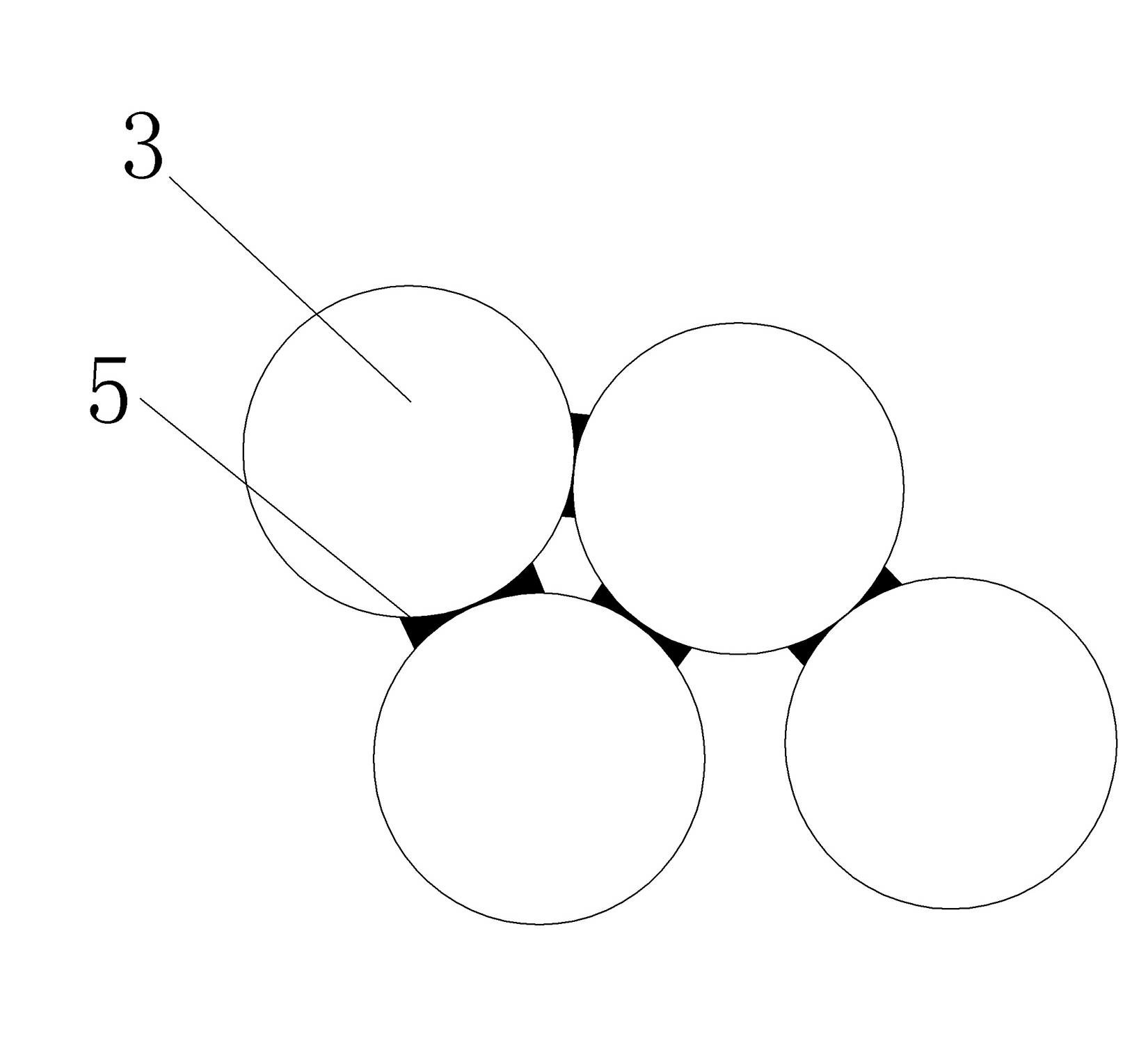
[0023] (2) Wet the ceramic balls 3 accumulated in the reaction vessel with water, and the excess water is discharged from the air inlet at the bottom. figure 2 As shown, there is adhesion water 5 in the contact part between the ceramic balls;
[0024] (3) Take 3kg of silicon tetrachloride, heat it to make it gasify, and pass it into the reaction vessel from the air inlet at the bottom, and react with the adhered water 5 in the contact part between the ceramic balls to form silicon dioxide, and at the same time release Hydrogen chloride gas...
Embodiment 2
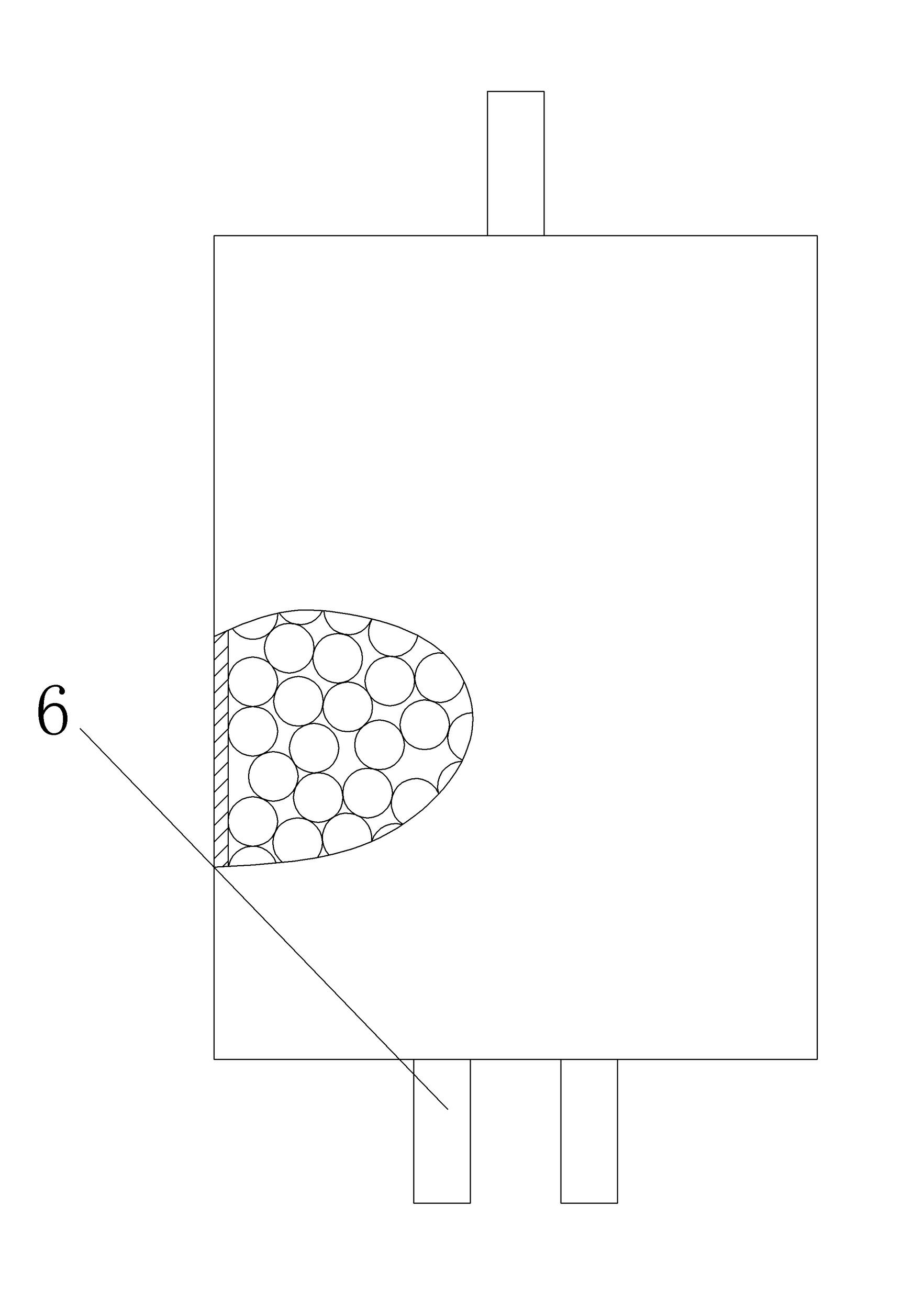
[0028] The silicon dioxide production method of the present embodiment is the same as embodiment 1, the difference is as image 3 As shown, the bottom of the reaction vessel is provided with a water vapor inlet 6, and while passing into silicon tetrachloride, water vapor lower than the reaction amount is passed into to ensure that silicon tetrachloride is excessive, and silicon tetrachloride enters the reaction vessel When it reacts with water vapor, with the reaction of silicon tetrachloride and adhering water, all the generated silicon dioxide is attached to the reaction carrier, and then the silicon dioxide is obtained through subsequent treatment, which can improve the reaction of silicon tetrachloride. Speed, improve the production efficiency of hydrochloric acid and silica.
[0029] The reaction time of this embodiment is 1 hour, and a total of 0.3 kg of water vapor is introduced to finally obtain 6.5 kg of hydrochloric acid (according to industrial concentrated hydrochl...
Embodiment 3
[0031] In this embodiment, the method for producing hydrochloric acid co-production silica with perlite as a reaction carrier, the specific steps are as follows:
[0032] (1) Pile up 10kg of perlite with an average particle size of 3-5mm in the reaction vessel;
[0033] (2) Wet the perlite accumulated in the reaction vessel with water, and the excess water is discharged from the air inlet at the bottom, and water is stored in the macropore structure of the perlite after wetting;
[0034] (3) Take 70kg of silicon tetrachloride, heat it to make it vaporize, and pass it into the reaction vessel from the air inlet at the bottom, react with the water stored in the perlite to form silicon dioxide, and the hydrogen chloride gas released from the reaction vessel The gas outlet on the top was discharged, absorbed by a container with a spray device, and the reaction took 6 hours to obtain 140kg of hydrochloric acid (according to industrial concentrated hydrochloric acid with a concentra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com