Ceramic precursor carbon-free polyborosilazane and synthesis method thereof
A technology for polyborosilazane and ceramic precursors, which is applied in the field of ceramic precursor carbon-free polyborosilazane and its synthesis, can solve the problem of high viscosity, no carbon-free polyborosilazane precursor synthesis method, ceramics Unsatisfactory yield and other problems, to achieve the effect of low viscosity, moderate and stable reaction, and good fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) 71g borane ammonia complex (H 3 B·NH 3 ) was dissolved in 3000g of diethylene glycol dimethyl ether, and then the solution was heated to 150°C, and the pyrolysis reaction started. Pass the escaped gas through a flask placed in an alcohol bath at 0°C to remove the solvent, cool the remaining gas to -78°C, and collect 53 g of liquid product. The obtained product was further purified by liquid nitrogen to obtain 45 g of relatively pure borazine.
[0021] (2) Add 1000mL pyridine and 100g halosilane H 2 SiCl 2 , the two react to form the by-product H 2 SiCl 2 ·xPy precipitated, passed excess ammonia gas (about 10L) at a flow rate of 100mL / min into the flask, and stopped the reaction after reflux at room temperature for 2h. A large amount of white precipitates were formed in the reaction mixture. After standing for 5 hours, filter under the protection of an inert atmosphere to obtain a pyridine solution of perhydropolysilazane. Distill under reduced pressure (1-3kPa...
Embodiment 2
[0024] (1) borane ammonia complex 71g (H 3 B·NH 3 ) was dissolved in 3500 g of triethylene glycol dimethyl ether, and then the solution was heated to 170°C. The pyrolysis reaction starts. The solvent was removed by passing the evolved gas through the flask placed in an alcohol bath at -10°C. The remaining gas was cooled to -78°C, and 52 g of liquid product was collected. The obtained product was further purified by liquid nitrogen to obtain 49.6 g of relatively pure borazine.
[0025] (2) Add 1500mL pyridine and 130gHSiCl 3 , the two react to form a white complex HSiCl 3 xPy. Pass excess ammonia gas into the flask at a flow rate of 100 mL / min, and stop the reaction after refluxing at 50° C. for 1 h. A large amount of white precipitate formed in the reaction mixture, which was filtered under an inert atmosphere after standing for 5 hours to obtain a pyridine solution of perhydropolysilazane. The solvent pyridine was removed by distillation under reduced pressure (pressu...
Embodiment 3
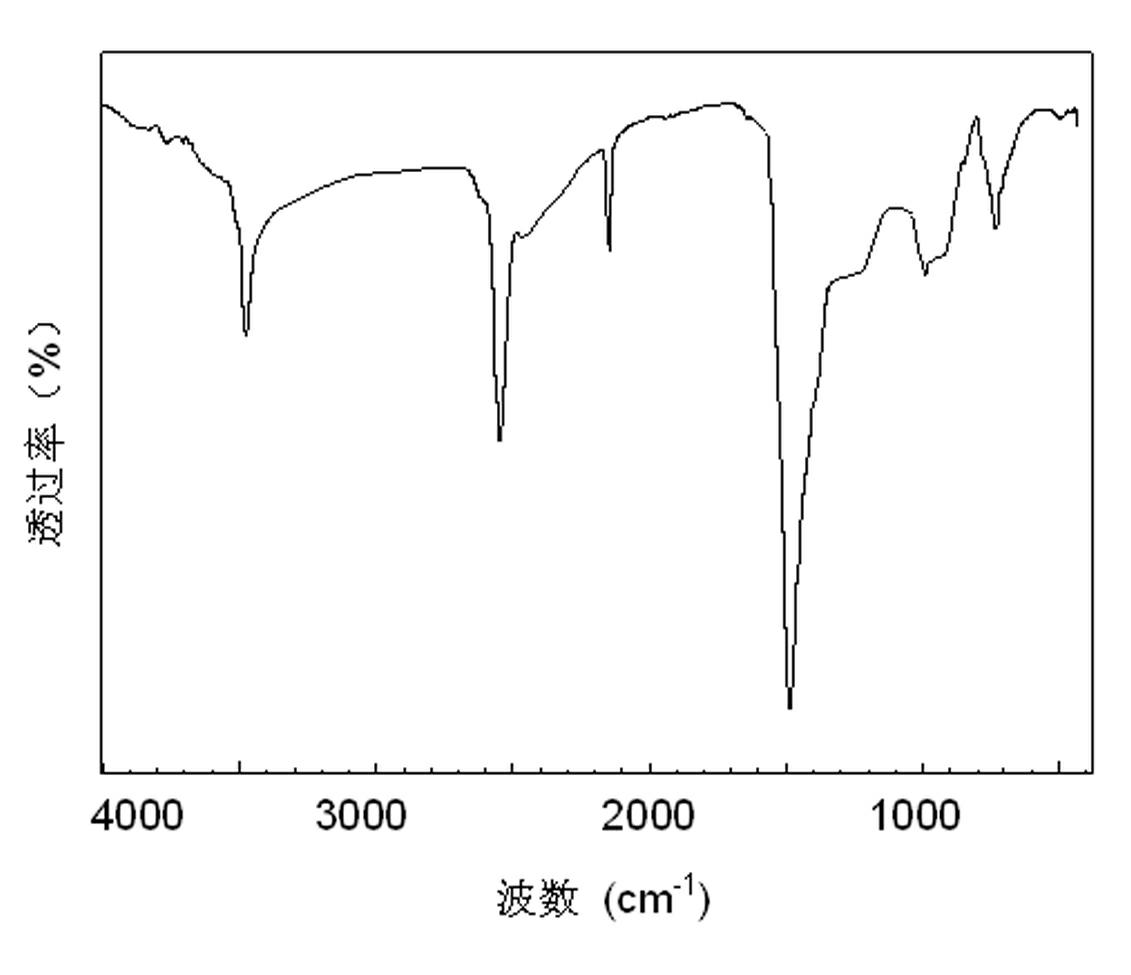
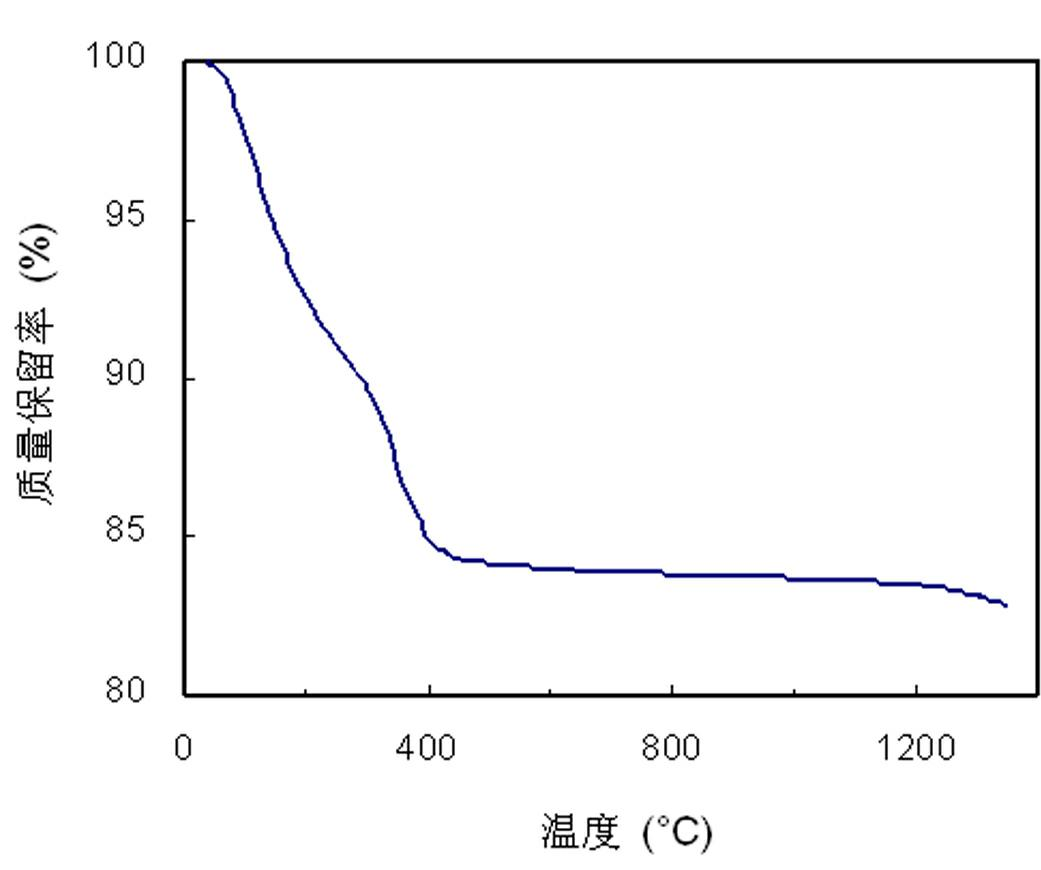
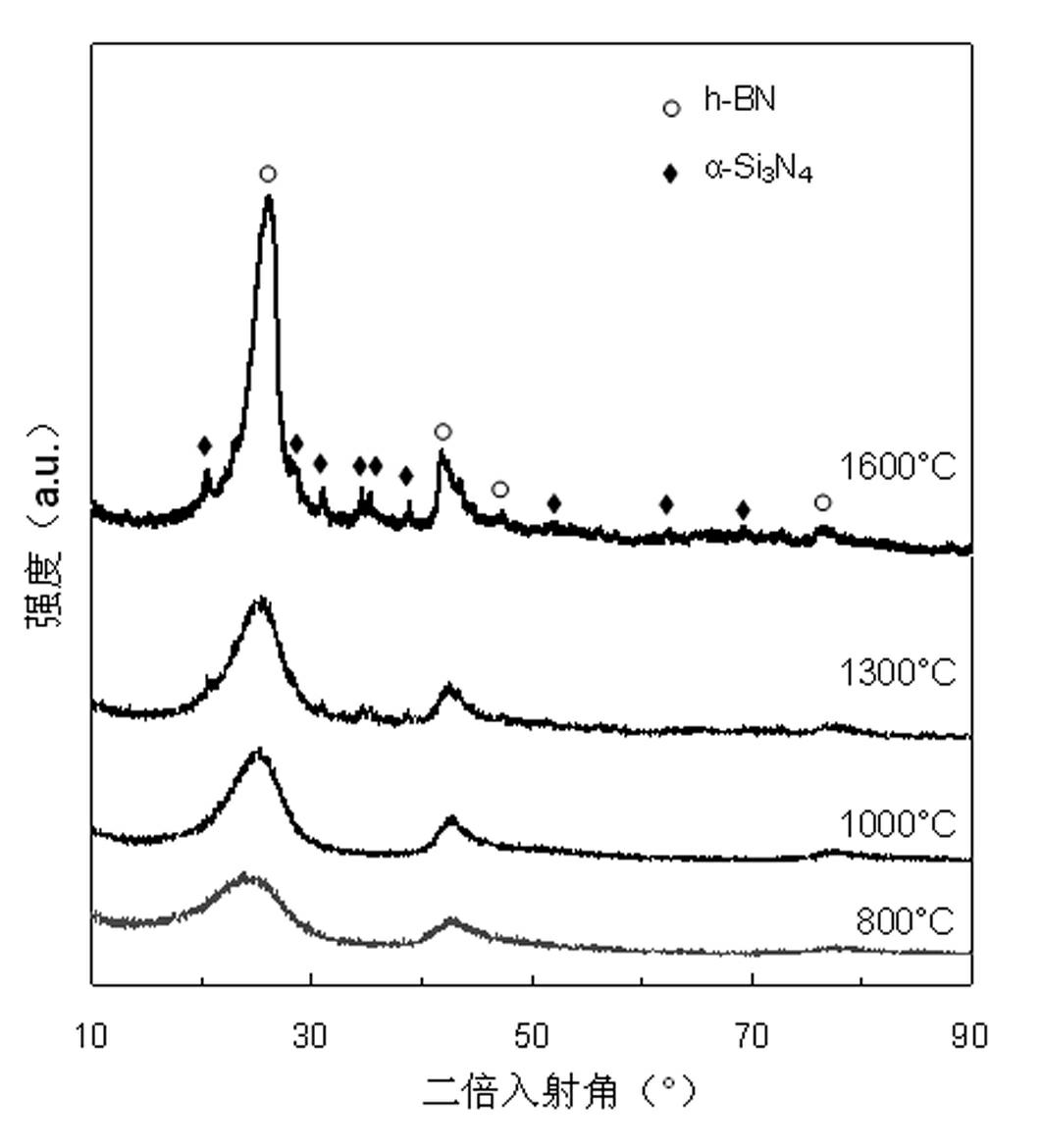
[0028] The carbon-free polyborosilazane of the present invention contains both Si-N bonds and B-N bonds in its structure, does not contain carbon and other impurity elements except H, and can be cracked to obtain pure silicon nitrogen boron ceramics. Its synthetic method is carried out as follows:
[0029] (1) borane ammonia complex 71g (H 3 B·NH 3 ) was dissolved in 2000 g of tetraethylene glycol dimethyl ether, and then the solution was heated to 200°C. The pyrolysis reaction starts. The solvent was removed by passing the evolved gas through the flask placed in an alcohol bath at -10°C. The remaining gas was cooled to -78°C, and 51.2 g of liquid product was collected. The obtained product was further purified by liquid nitrogen to obtain 50.1 g of relatively pure borazine.
[0030] (2) Add 1000mL pyridine and 80g H 3 SiCl, the two react to form a white complex H 3 SiCl xPy. Pass excess ammonia gas into the flask at a flow rate of 100 mL / min, and stop the reaction aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com