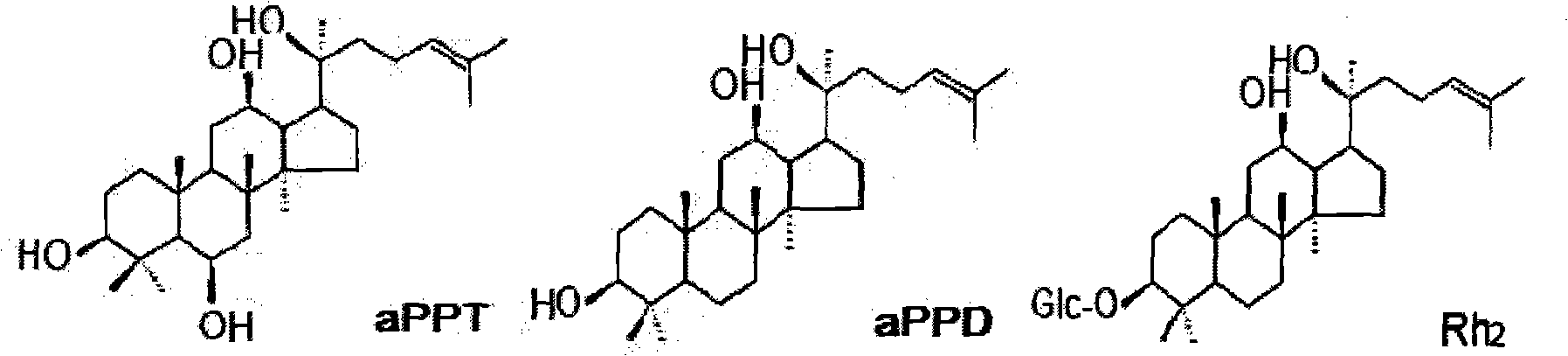
Dammarane aglycon compound and application thereof
A technology for dammarane aglycone and complex, which is applied to the dammarane aglycone complex and its application field, can solve the problems such as the prevention and protection of myelosuppression that have not been reported, and achieves an increase in peripheral blood levels and colonies. Quantity, clear effect of protective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
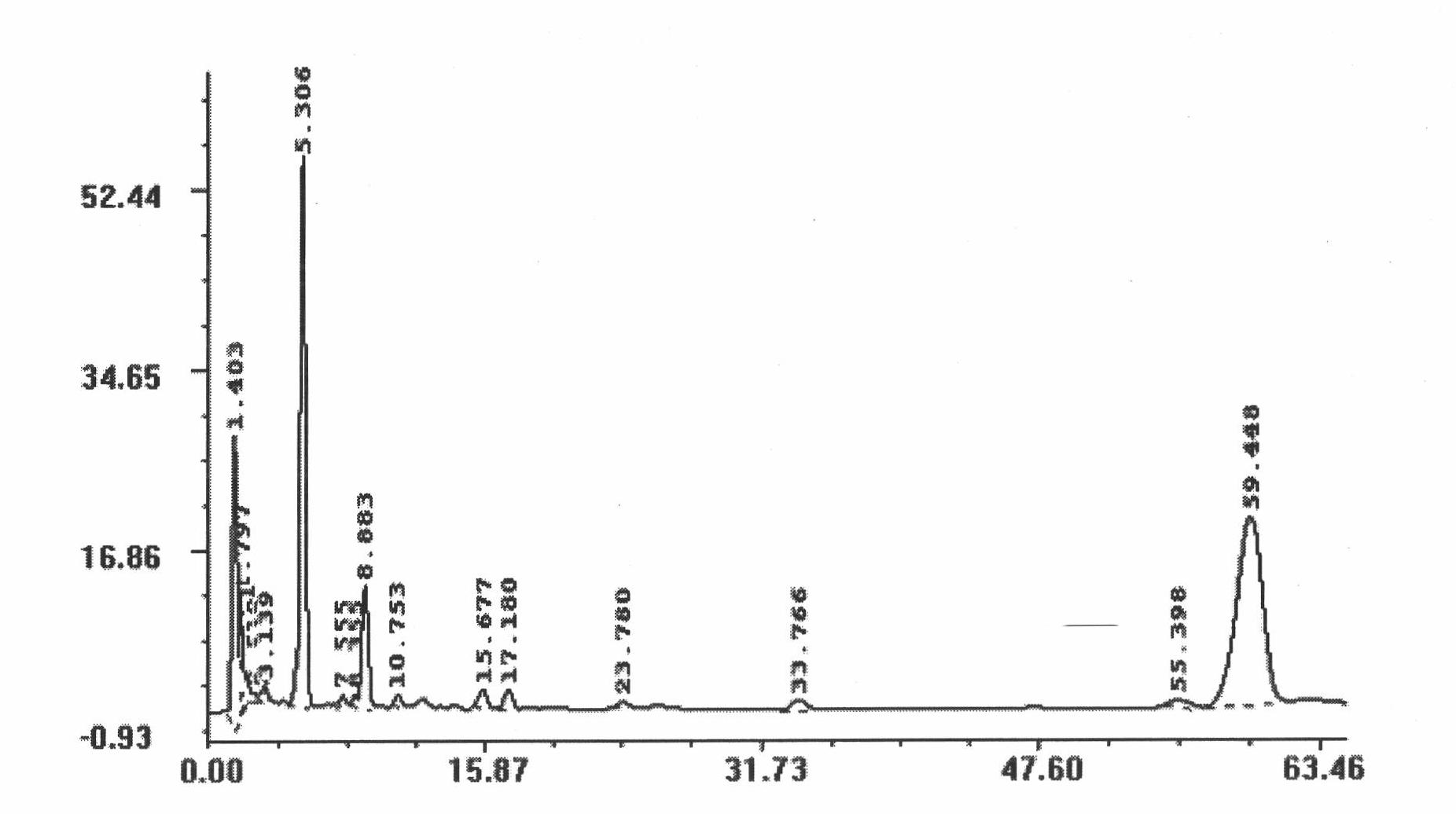
[0021] Effects of dammarane aglycon complex on the colony number of hematopoietic progenitor cells in myelosuppressed mice
[0022] Cyclophosphamide 200mg / kg was used in the experiment, and mice were injected intraperitoneally once to establish a model. Three days after the model was established, bone marrow cells were collected for colony culture, and different concentrations of dammarane aglycon complexes were added to the culture system. The experimental results are shown in Table 1. Among them, the mice in the model group were not administered externally with the dammarane aglycon complex, and the mice in the experimental group were administered externally with different concentrations of the dammarane aglycon complex.
[0023] Table 1 The effect of dammarane aglycon complex on the number of hematopoietic progenitor cell colonies in myelosuppressed mice ( n=3)
[0024]
[0025] Note: * means P<0.05; ** means P<0.01, compared with the model group.
[0026] Experiment...
Embodiment 2
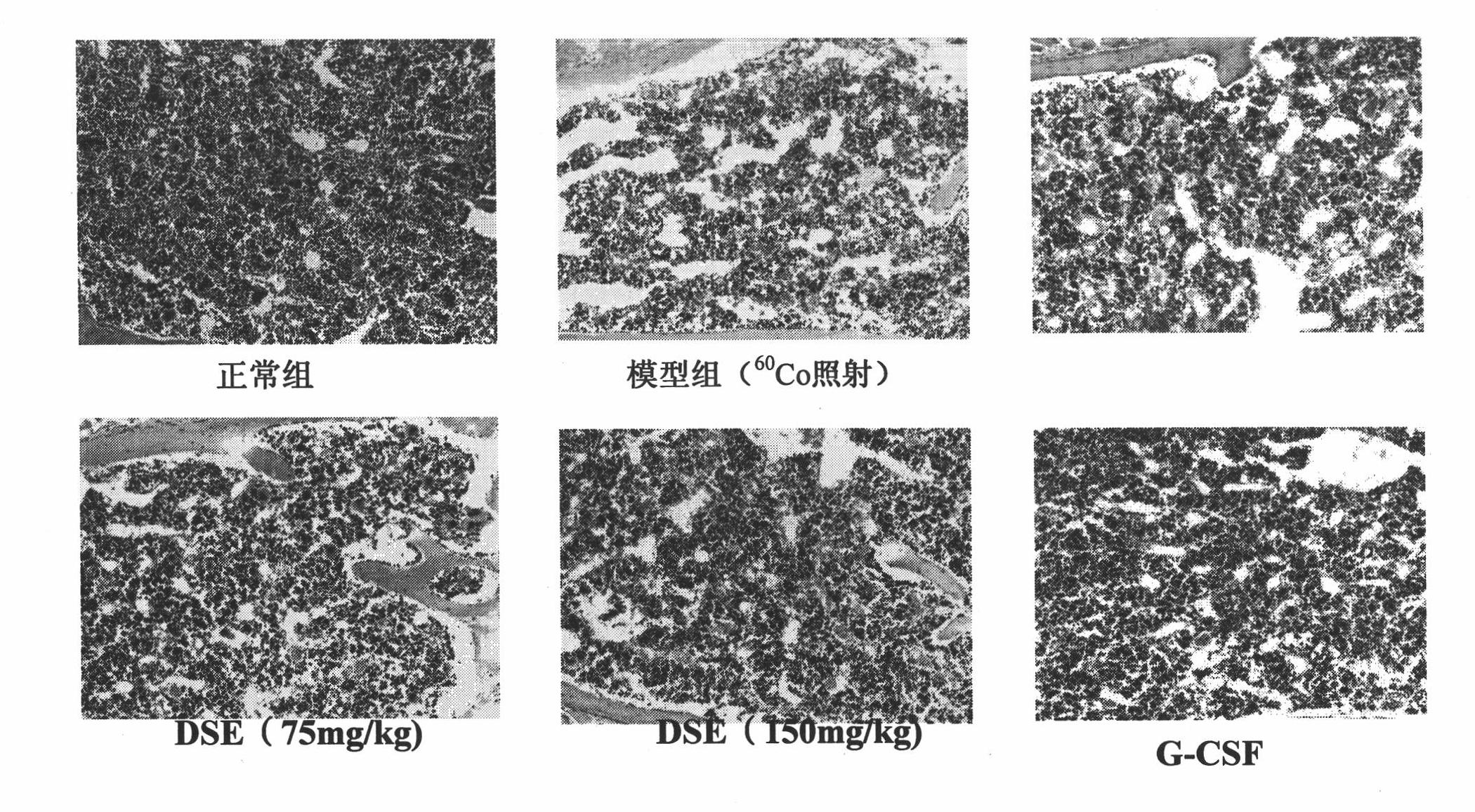
[0028] Effects of Oral Administration of Dammarane Aglycone Complex on Peripheral Blood of Mice with Myelosuppression Induced by Cyclophosphamide
[0029] Experimental settings Control group, model group, low-dose group (oral administration of dammarane aglycon complex 37.5mg / kg), medium-dose group (oral administration of dammarane aglycon complex 75mg / kg) and high-dose group (Orally administered dammarane aglycon complex 150 mg / kg group), and the positive drug group. Except for the control group, all other groups were intraperitoneally injected with cyclophosphamide (CTX) 200 mg / kg to establish models, and half an hour after the injection, 0.2 ml / 10 g of dammarane aglycone complex of the corresponding concentration was intragastrically administered every day. The G-CSF test solution, 0.1ml / 10g, was injected subcutaneously in the control group and 0.2ml / 10g of distilled water in the control group and the model group for 5 consecutive days. The experimental results are shown i...
Embodiment 3
[0039] Effects of dammarane aglycon complex on hematopoietic progenitor cells in mice with myelosuppression induced by cyclophosphamide
[0040] On the day of the experiment, the mice were intraperitoneally injected with cyclophosphamide to establish a model, and the administration group was given a small dose (37.5 mg / kg), a medium dose (75 mg / kg) and a large dose (150 mg / kg) of dammarane aglycone complex by intragastric administration immediately after the model was established. / kg), the normal group and the model group were given the same volume of distilled water, the administration volume was 0.2ml / 10g, and the administration was continued for 10 days. The results are shown in Table 3.
[0041] Table 3 Effects of dammarane aglycon complex on hematopoietic progenitor cells in mice with bone marrow suppression induced by cyclophosphamide ( n=6)
[0042]
[0043] Note: ## means p<0.01, compared with the normal group; * means p<0.05, ** means p<0.01, compared with the m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com