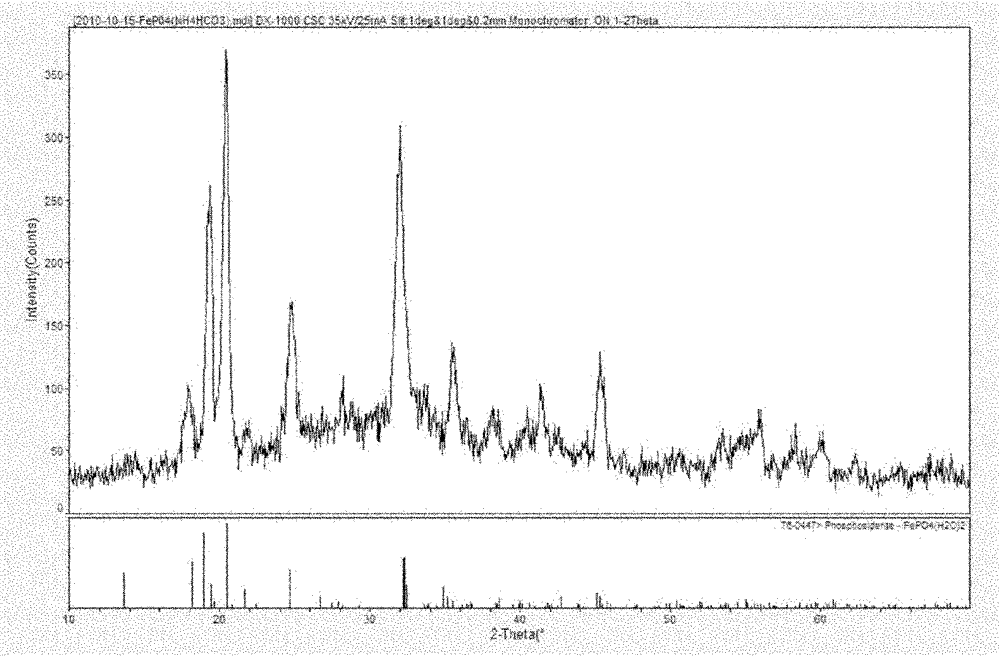
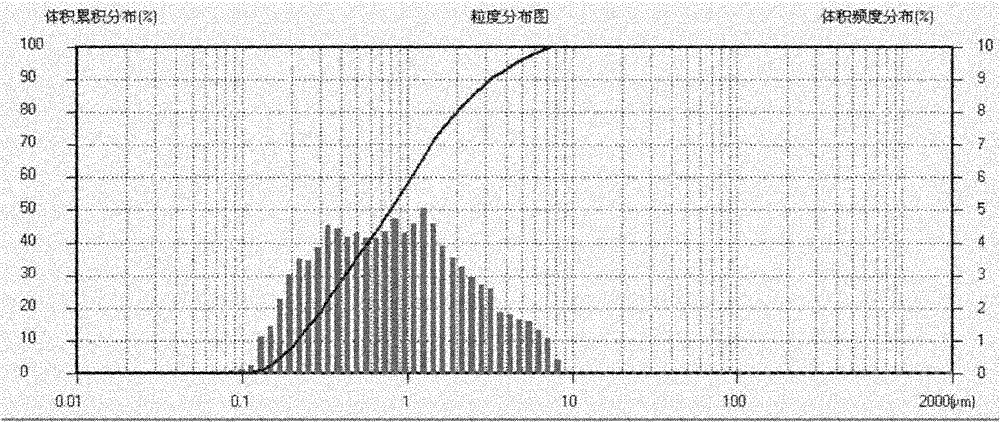
High-purity iron phosphate used for producing lithium ion battery positive-pole material and preparation method thereof
A technology for lithium-ion batteries and cathode materials, which is applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of long synthesis cycle, high production cost, high energy consumption and price, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1 : prepare ferric orthophosphate hydrate according to the present invention
[0069] Purify NH with pH=8, 1.5mol / L 4 HCO 3 The solution was slowly added to 300 mL of refined FeSO4 with pH=3 and Fe concentration of 4.579% under constant stirring. 4 solution, while using a pH meter to detect the pH of the reaction mixture solution, when the pH=7, stop adding the NH 4 HCO 3 Solution, after the bubbles in the reaction system completely disappear, let stand, filter and separate to obtain the filtrate and filter cake, and recover ammonium sulfate, FeCO 3 The filter cake was washed with deionized water until it was washed with Ba(NO 3 ) 2 The solution detection filtrate has no white turbidity;
[0070] Then, the washed FeCO 3 The precipitate was transferred to a beaker, and phosphoric acid with a concentration of 85% was added according to the molar ratio of phosphorus to iron of 1:1, and the stirring was continued for half an hour. During the stirring proces...
Embodiment 2
[0073] Example 2 : prepare ferric orthophosphate hydrate according to the present invention
[0074] Purify NaHCO with pH=8, 2mol / L 3 The solution was slowly added to 300 mL of refined FeSO4 with pH=3 and Fe concentration of 4.579% under constant stirring. 4 In solution, detect the pH of reaction mixture solution with pH meter simultaneously, when pH=6, stop adding described NaHCO 3 Solution, after the bubbles in the reaction system completely disappear, let stand, filter and separate to obtain the filtrate and filter cake, and recover ammonium sulfate, FeCO 3 The filter cake was washed with deionized water until it was washed with Ba(NO 3 ) 2 Solution detection filtrate no white turbidity appears;
[0075] Then, the washed FeCO 3 Transfer the precipitate to a beaker, add phosphoric acid with a concentration of 85% according to the molar ratio of phosphorus to iron 1.5:1, continue to stir for half an hour, heat up to 50 ° C during the stirring process, and let the FeCO ...
Embodiment 3
[0078] Example 3 : prepare ferrous orthophosphate dihydrate according to the present invention
[0079] Purify Na with pH=8, 2.5mol / L 2 CO 3 The solution was slowly added to 300 mL of refined FeCl with pH=2 and Fe concentration of 6.585% under constant stirring. 2 In solution, detect the pH of reaction mixture solution with pH meter simultaneously, when pH=8, stop adding described Na 2 CO 3 Solution, after the bubbles in the reaction system completely disappear, let stand, filter and separate to obtain the filtrate and filter cake, and recover ammonium sulfate, FeCO 3 The filter cake was washed with deionized water until AgNO 3 Solution detection filtrate no white turbidity appears;
[0080] Then, the washed FeCO 3 Transfer the precipitate to a beaker, add phosphoric acid with a concentration of 85% according to the molar ratio of phosphorus to iron 1.3:1, continue to stir for half an hour, heat up to 50 ° C during the stirring process, and let the FeCO 3 The precipitat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com