Everolimus solid oral medicinal composition
A technology of everolimus and composition, applied in the direction of drug combination, antineoplastic drugs, pharmaceutical formulations, etc., can solve the problems of increasing the complexity of the operation process, increasing the cost, and the existence of potential safety hazards, so as to improve the solubility and stability of the drug non-toxicity, less dust pollution, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
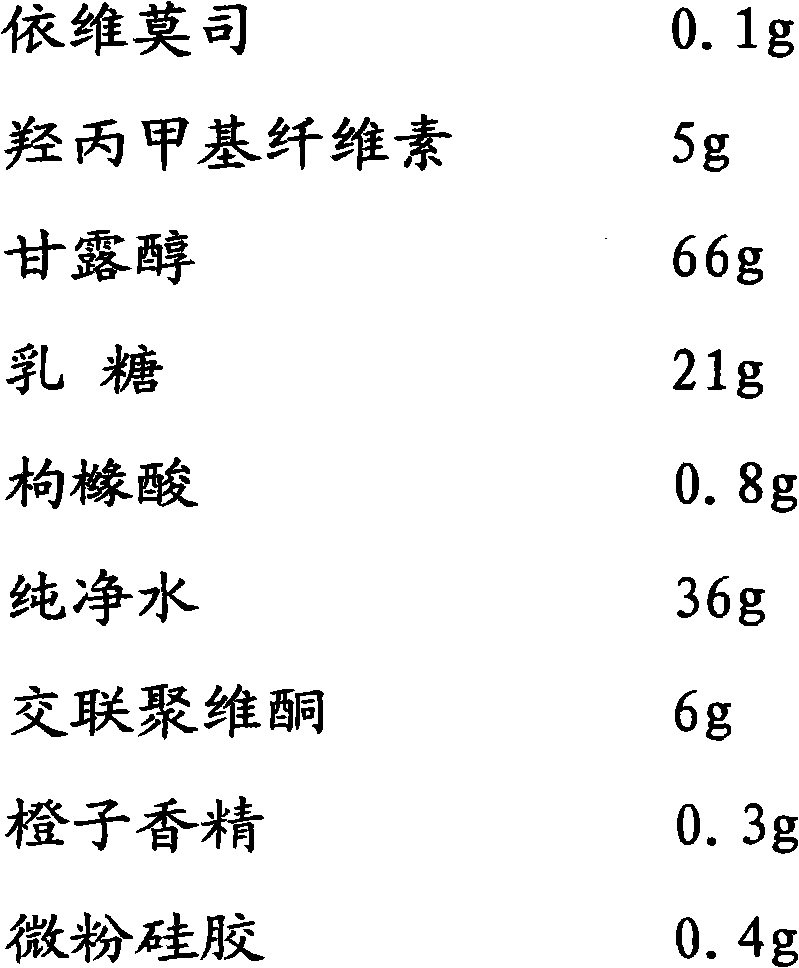
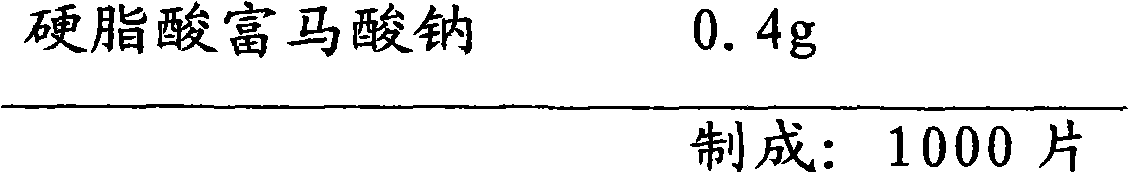
[0036] A prescription for orally disintegrating tablets of everolimus solid oral pharmaceutical composition:
[0037]
[0038]
[0039] The manufacturing process of this embodiment is as follows:
[0040] Weigh the raw and auxiliary materials of the prescribed amount, pass everolimus through a 100-mesh sieve, microcrystalline cellulose, mannitol, lactose, citric acid, crospovidone, and orange essence pass through a 80-mesh sieve, micropowder silica gel, stearin Sodium fumarate is passed through a 100-mesh sieve. Take the prescribed amount of mannitol and put it in the GPCG1.1 fluidized bed coating machine equipped with a bottom spray, adjust the inlet air temperature to 40°C, the outlet air temperature to 25°C, and the material temperature to 25°C to make the mannitol evenly distributed in the fluidized bed. boiling. Adjust the atomization pressure of the spray gun to 2 bar, and the speed of the peristaltic pump to 20 rpm, evenly disperse the prescribed amount of evero...
Embodiment 2
[0042] A prescription for dispersible tablets of everolimus solid oral pharmaceutical composition:
[0043]
[0044]
[0045] The manufacturing process of this embodiment is as follows:
[0046] The raw and auxiliary materials of the prescription amount were weighed, everolimus was passed through a 100-mesh sieve, mannitol, lactose, citric acid, hydroxypropyl cellulose, crospovidone, and banana essence were respectively passed through a 80-mesh sieve, micronized silica gel, hard Magnesium fatty acid passed through a 100-mesh sieve. Take the prescribed amount of mannitol and put it in the GPCG1.1 fluidized bed coating machine equipped with a bottom spray, adjust the inlet air temperature to 40°C, the outlet air temperature to 25°C, and the material temperature to 25°C to make the mannitol evenly distributed in the fluidized bed. boiling. Adjust the atomization pressure of the spray gun to 2 bar, and the speed of the peristaltic pump to 20 rpm, evenly disperse the prescr...
Embodiment 3
[0048] A prescription for a chewable tablet of everolimus solid oral pharmaceutical composition:
[0049]
[0050]
[0051] The manufacturing process of this embodiment is as follows:
[0052] Weigh the raw and auxiliary materials of the prescription amount, everolimus is passed through a 100-mesh sieve, microcrystalline cellulose, mannitol, povidone, lactose, sodium citrate, pregelatinized starch, sodium carboxymethyl starch, apple essence, Peppermint essence is passed through a 80-mesh sieve, and stearic acid and magnesium micropowder silica gel are passed through a 100-mesh sieve. Take the prescribed amount of mannitol and put it in the GPCG1.1 fluidized bed coating machine equipped with a bottom spray, adjust the inlet air temperature to 40°C, the outlet air temperature to 25°C, and the material temperature to 25°C to make the mannitol evenly distributed in the fluidized bed. boiling. Adjust the atomization pressure of the spray gun to 2 bar, and the speed of the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com