Citicoline sodium glucose injecta and preparation process thereof
A technology of glucose injection and citicoline sodium is applied in the directions of medical preparations with non-active ingredients, medical preparations containing active ingredients, drug delivery, etc. The effects of quality stability, wake-up promotion, and brain function recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation prescription:
[0030] Main ingredient: Citicoline Sodium 2.5g
[0031] Isotonic agent: glucose 50g
[0032] Stabilizer: malic acid 0.5g
[0034] pH regulator 10% sodium hydroxide appropriate amount
[0035] Add water for injection to 1000ml
[0036] Preparation Process:
[0037] (1) Add an appropriate amount of water for injection into the concentrated preparation tank, heat, put in the prescribed amount of glucose, malic acid, and sodium sulfite, stir to dissolve, add the prescribed amount of activated carbon for injection, boil for 15 minutes, and decarbonize the solution through a titanium rod filter , the filtrate is pumped into the dilute distribution tank.
[0038] (2) Put the prescribed amount of citicoline sodium and an appropriate amount of 10% sodium hydroxide solution into the dilute preparation tank, add water for injection to the full amount, and stir evenly.
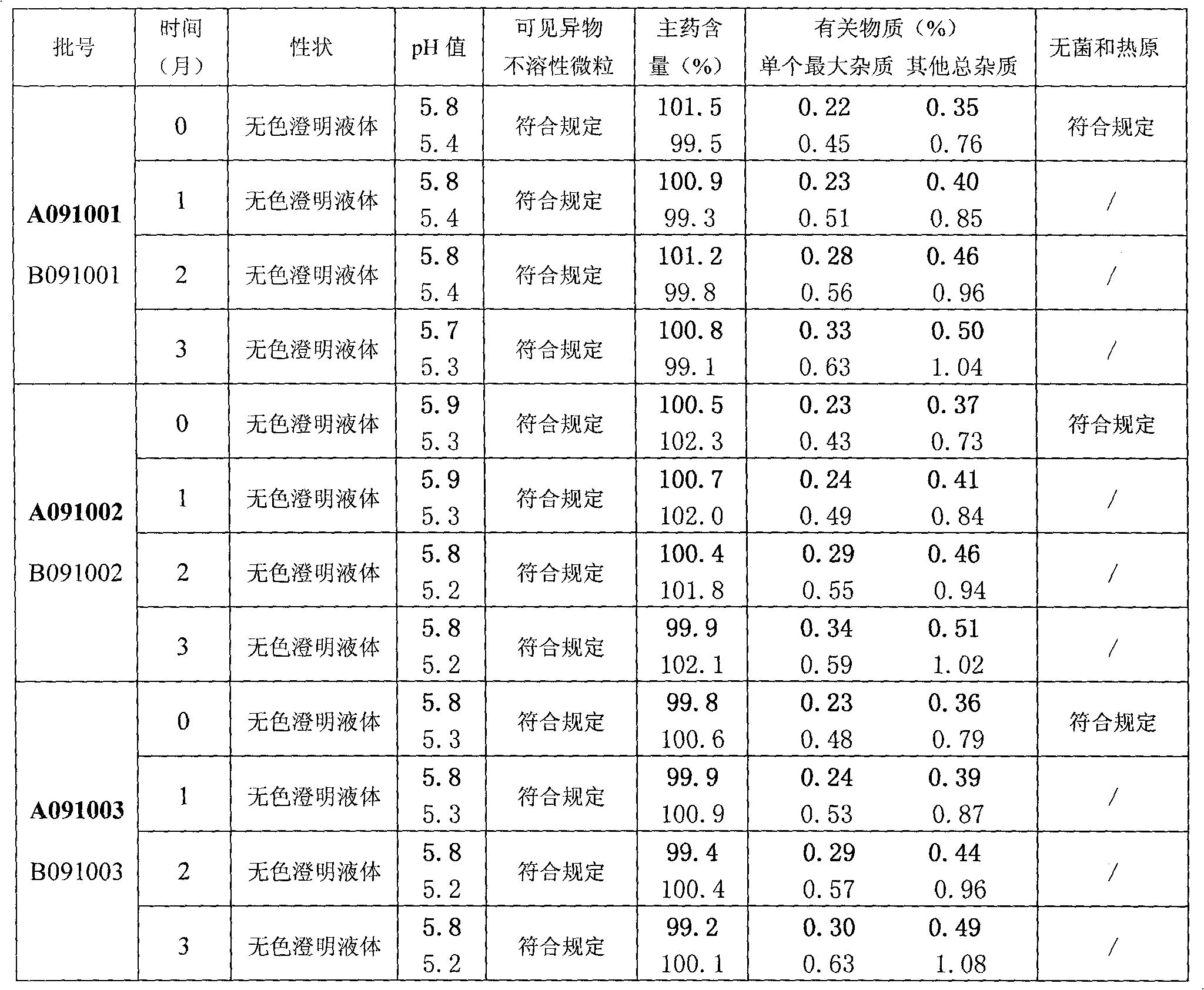
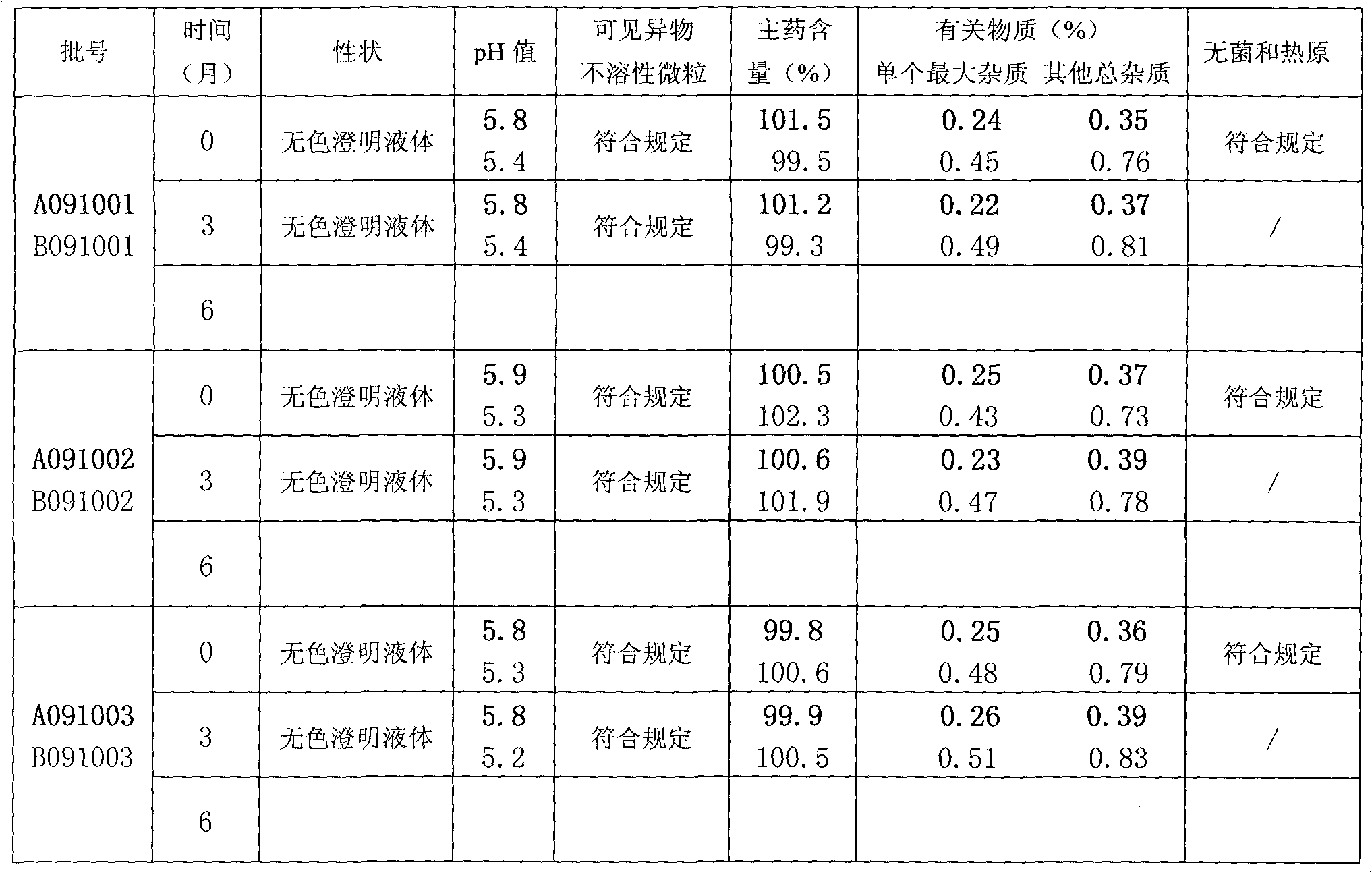
[0039] (3) After testing the liquid content and ...
Embodiment 2
[0042] Preparation prescription:
[0043] Main ingredient: Citicoline Sodium 2.5g
[0044] Isotonic agent: glucose 50g
[0045] Stabilizer: sodium malate 0.5g
[0047] pH regulator 10% sodium hydroxide appropriate amount
[0048] Add water for injection to 1000ml
[0049] Prepared by the technique of Example 1.
Embodiment 3
[0051] Preparation prescription:
[0052] Main ingredient: Citicoline Sodium 2.5g
[0053] Isotonic agent: glucose 50g
[0054] Stabilizer: malic acid 0.5g
[0055] Sodium metabisulfite 0.5g
[0056] pH regulator 10% sodium hydroxide appropriate amount
[0057] Add water for injection to 1000ml
[0058] Prepared by the technique of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com