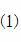Preparation of loaded Grubbs catalyst and application of loaded Grubbs catalyst
A catalyst and supported technology, applied in the field of chemical catalytic materials and chemical applications, can solve problems such as the reduction of catalytic activity, and achieve the effects of simplifying post-treatment, reducing heavy metal pollution, and improving catalytic activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] a. Preparation of aminated mesoporous materials
[0028] Vacuumize the SBA-15 mesoporous material at 90°C for 4 hours and cool to room temperature, then take 2.5g of SBA-15 inorganic mesoporous material and mix it with 43ml of anhydrous toluene under the protection of argon, then add 1.25g of 3-amino Propyltriethoxysilane, then heated to 110°C under reflux and stirred for 24 hours, filtered after the reaction, the filtrate was washed with 30ml of toluene, 30ml of ethanol and 30ml of acetone, collected, and dried in vacuo to obtain light yellow solid amino FeSBA-15 mesoporous material 2.8g, its 3-aminopropyltriethoxysilane, ethanol and acetone are chemically pure.
[0029] b. Preparation of amidated mesoporous materials
[0030] At room temperature and under argon protection, mix 1.0g 3-3-vinyl-4-isopropoxyphenyl-propionic acid with 0.98g dicyclohexylcarbodiimide, 0.16g 4-dimethylaminopyridine, 0.075 g of p-toluenesulfonic acid and 48ml of N,N-dimethylformamide were mi...
Embodiment 2
[0037] a. Preparation of aminated mesoporous materials
[0038] at -5 o C and under argon protection, mix 2.5g FDU-14 mesoporous material with 12ml chloromethyl ether, add 3.0g anhydrous aluminum trichloride in three batches, stir at room temperature for 12 hours, then cool the reaction mixture to 0 o After C, filter, and the filtrate is washed successively with 30 parts by weight of water and 10 parts by weight of acetone and collected, and after vacuum drying, the brown solid is chlorinated FDU-14 mesoporous material, and its chloromethyl ether, anhydrous aluminum trichloride and acetone are chemically pure;
[0039] At room temperature and under the protection of argon, mix 2.5g of the above-prepared chlorinated FDU-14 mesoporous material with 12ml of anhydrous ethylenediamine, stir at 80°C for 24 hours, filter after the reaction, and use the filtrate in turn After washing with 100ml of water and 50ml of acetone, it was collected and dried in vacuo to obtain a brown soli...
Embodiment 3
[0048] The specific application of SBA-15 first-generation supported Grubbs catalyst for N, N-diallyl-4-methylbenzenesulfonamide catalyzed ring-closing olefin metathesis reaction (RCM) will be further described in detail for the present invention :
[0049] Under the protection of argon, 0.36 g of the first-generation supported Grubbs catalyst of SBA-15 mesoporous material supported phenylmethylene-bistricyclohexylphosphine-ruthenium dichloride was mixed with N in a dry reaction tube. , N-diallyl-4-methylbenzenesulfonamide 0.2g and dichloromethane 4ml mixed, stirred and heated to 38 o C, TLC monitoring to the end of the reaction, then suction filtration, the filtrate was concentrated and column chromatography to obtain a white solid 2,5-dihydro-1-p-toluenesulfonylpyrrole, the yield was 98%, filtered out The catalyst was collected after being washed three times with 8ml of dichloromethane, and the catalyst was recovered for recycling. N, N-diallyl-4-methylbenzenesulfonamide wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com