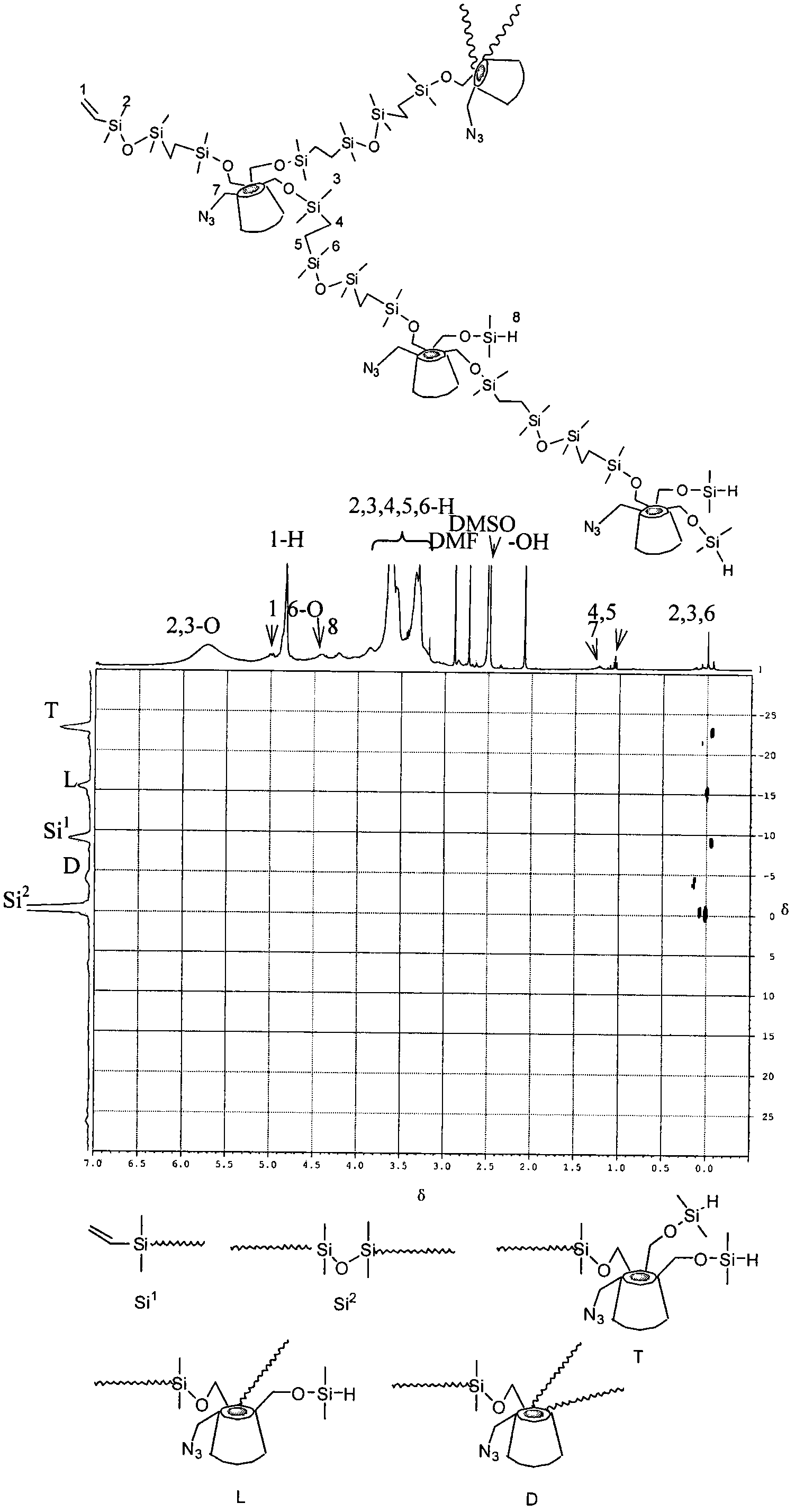
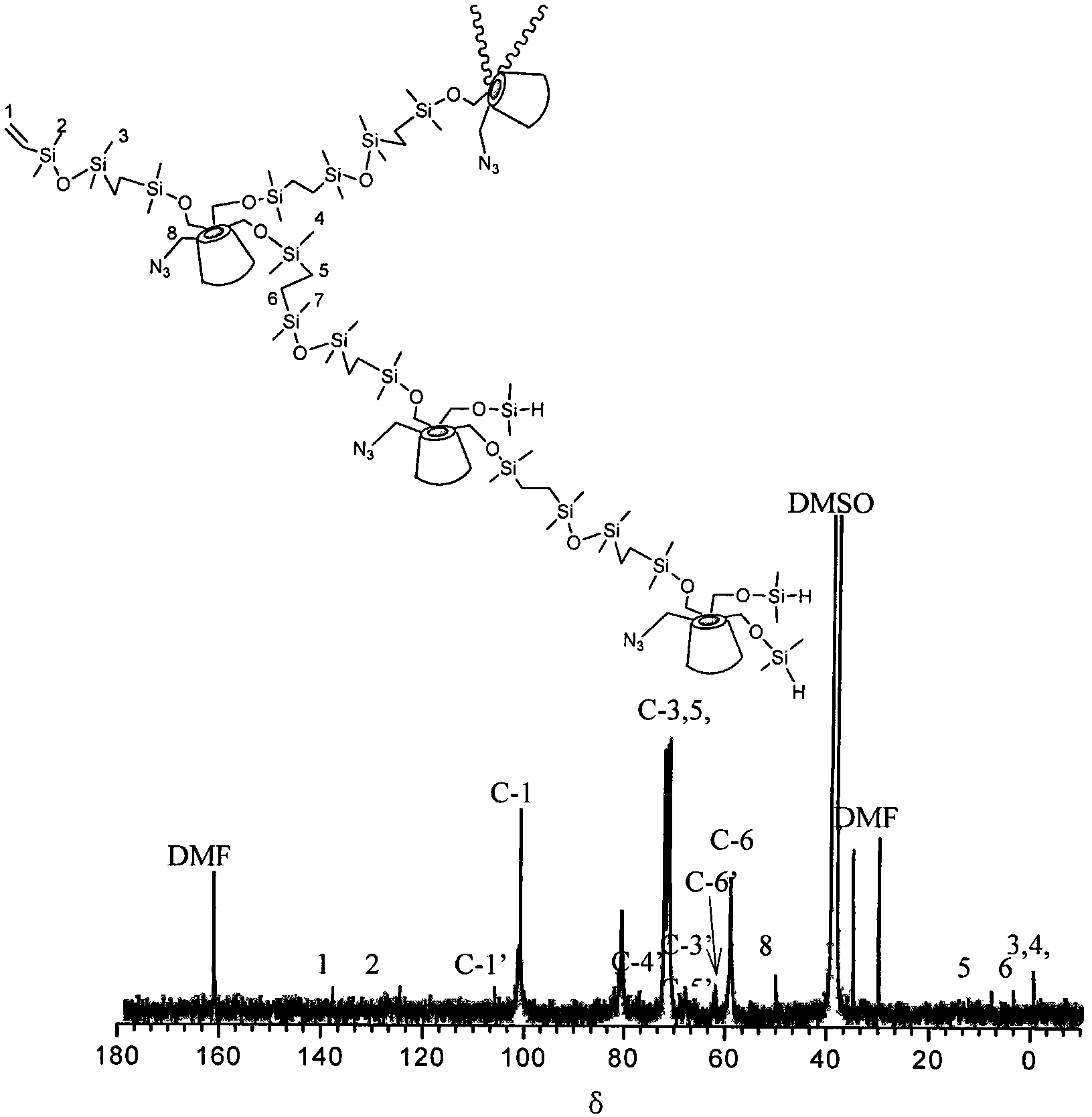
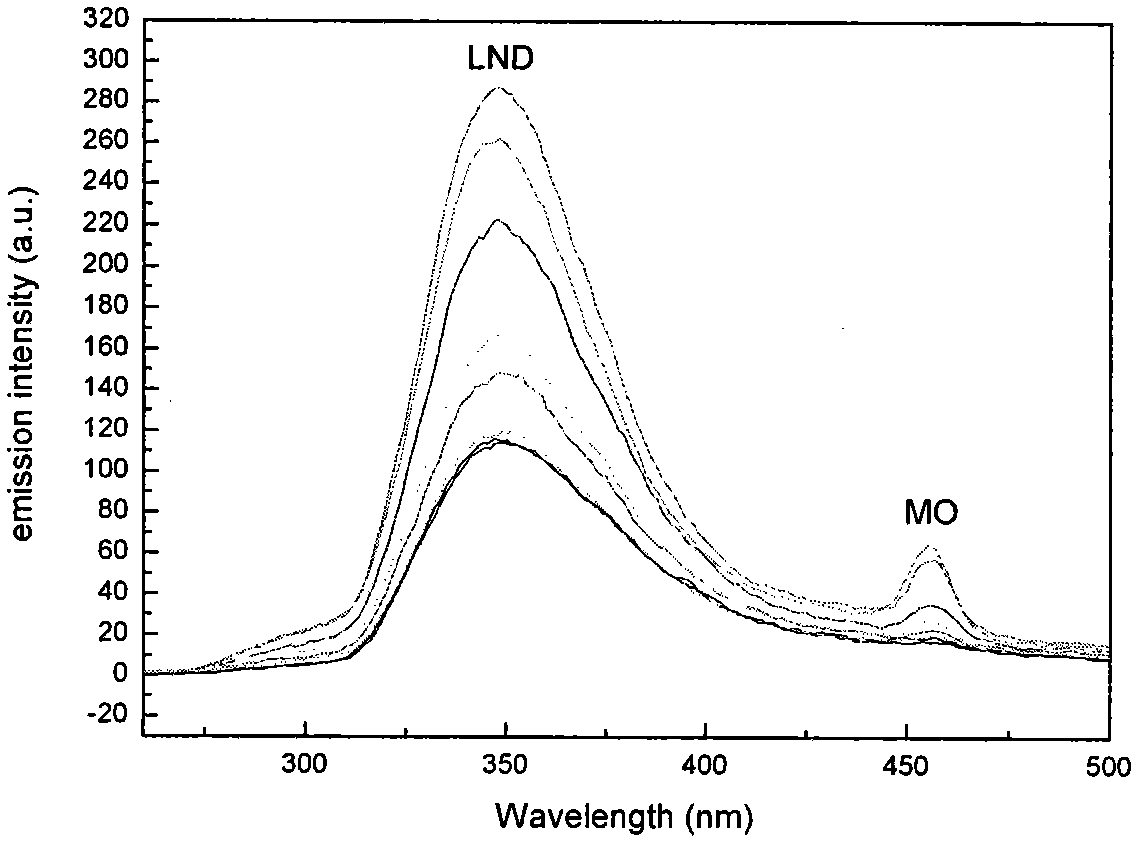
Preparation method of hyperbranched poly(beta-cyclodextrin) containing azide group
A technology of hyperbranched polycyclodextrin, which is applied in the field of preparation of hyperbranched polycyclodextrin, can solve the problem of single inclusion guest molecules, and achieve the effect of simplified steps and high controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Weigh 3g of six-position monosubstituted p-tosylated β-cyclodextrin, 0.193g of potassium iodide, and 1.52g of sodium azide in a dry single-necked flask, add 10mL of N,N-dimethylformamide as a solvent, Fully dissolve for 0.5 h under nitrogen protection and magnetic stirring. After dissolving the above substances, the resulting system was sealed with nitrogen protection and continued to stir and react at 80° C. for 26 h. Add 30 mL of distilled water to the solution after the reaction and continue to stir for 20 min, and filter the obtained turbid solution with a G4 sand core funnel to remove insoluble matter to obtain a transparent solution. Gradually add 60 mL of acetone-water blend solvent (the volume ratio of acetone to water is 2:1) to the transparent solution, slowly precipitate the precipitate, put it in the refrigerator at 4°C for 24h, filter out the solid product, and dry it in vacuum at 25°C In 3 days, 2.4 g of six-position monosubstituted azide β-cyclodextrin m...
Embodiment 2
[0026] Add 6g of six-position monosubstituted p-toluenesulfonylated β-cyclodextrin, 0.386g of potassium iodide, 3.05g of sodium azide and 15mL of N,N-dimethylformamide into a dry one-necked flask, under nitrogen protection, magnetic stirring Fully dissolved under the conditions of 0.5h. After dissolving the above substances, the resulting system was sealed with nitrogen protection and continued to stir and react at 85° C. for 25 h. After the reaction was completed, 60 mL of distilled water was added to the solution and continued to stir for 25 min. The obtained turbid solution was filtered with a G4 sand core funnel to remove insoluble matter to obtain a transparent solution. Gradually add 120mL of acetone-water blend solvent (the volume ratio of acetone to water is 2:1) to the transparent solution, and the precipitate slowly precipitates out, and the solid product is filtered out after standing at 4°C in the refrigerator for 24h, and the obtained solid product is 30°C Vacuum...
Embodiment 3
[0030] Add 10g of six-position monosubstituted p-toluenesulfonylated β-cyclodextrin, 0.643g of potassium iodide, 5.07g of sodium azide and 30mL of N,N-dimethylformamide into a dry single-necked flask, under nitrogen protection, magnetic stirring Fully dissolved under the conditions of 0.5h. After dissolving the above substances, the resulting system was sealed with nitrogen protection and continued to stir and react at 90° C. for 24 h. Add 100 mL of distilled water to the solution after the reaction, continue to stir for 30 min, filter the obtained cloudy solution with a G4 sand core funnel to remove insoluble matter, and obtain a transparent solution. Gradually add 180 mL of acetone-water blend solvent (the volume ratio of acetone to water is 2:1) to the transparent solution, and precipitate slowly. After standing in the refrigerator at 4°C for 24h, the solid is filtered out, and vacuum-dried at 30°C for 5 Tian obtained 7.1g of six-position monosubstituted azide β-cyclodextr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com