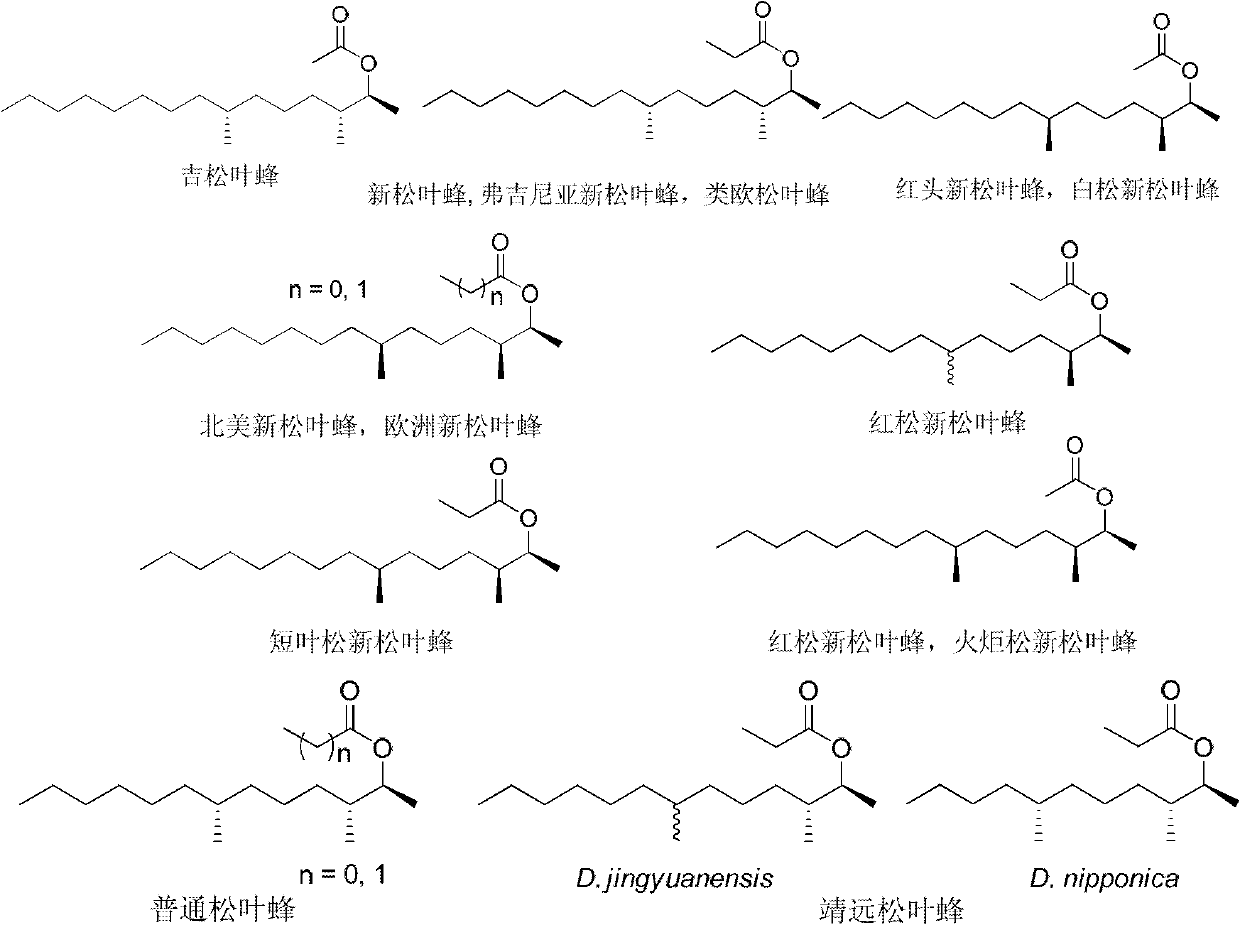
Method for synthesizing high-enantiomer-purity diprionidae pheromone and stereo isomer
A technology of stereoisomers and synthesis methods, which is applied in the field of synthesis of high-enantiomer pure pine sawfly sex pheromones and stereoisomers, can solve the problem of low activity and achieve high enantiomeric purity and good application foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
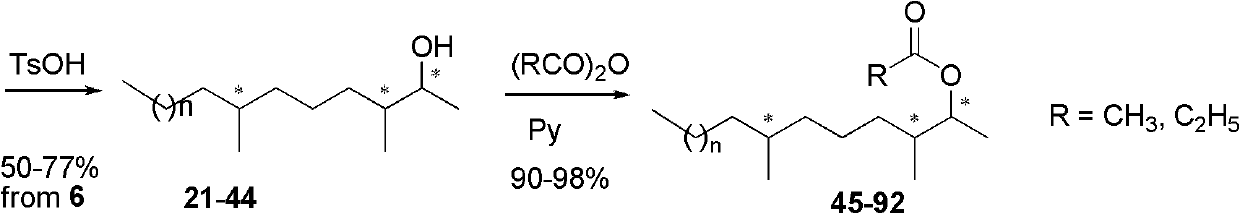
Examples
Embodiment 1
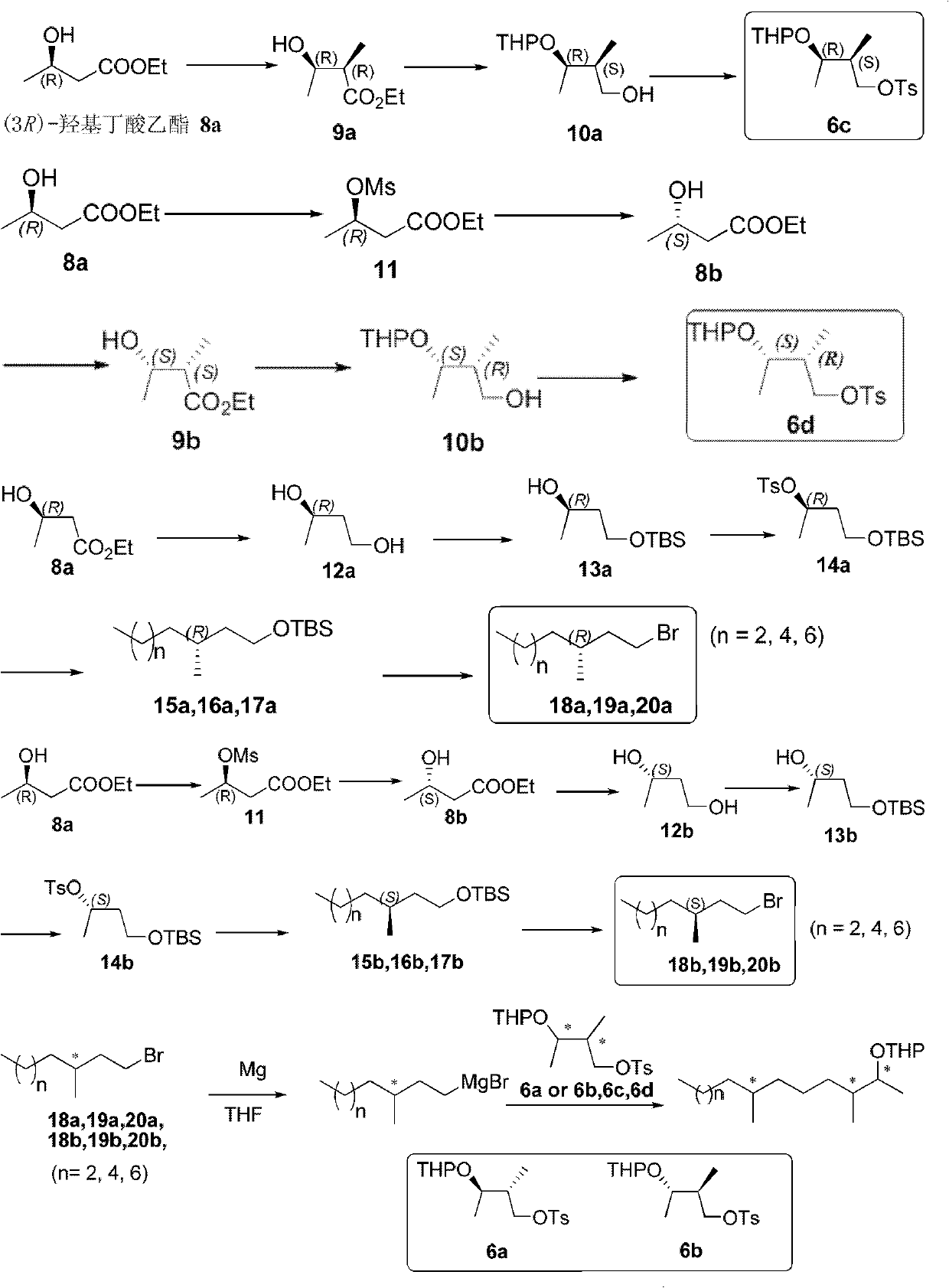
[0042] Step 1 Synthesis of (3R)-Methanesulfonate Butyrate Ethyl 11
[0043] 300 mL of dichloromethane was added to a single-necked flask containing ethyl (3R)-hydroxybutyrate (20.29 g, 153.5 mmol). Under ice bath, pyridine (24.3mL, 184.2mmol) and methanesulfonyl chloride (13.74g, 176.5mmol) were added dropwise to the solution in the single-necked flask. After stirring for 15 min at the same temperature, the mixture was filtered with suction to remove pyridine hydrochloride. The filtrate was concentrated under reduced pressure and subjected to flash column chromatography (ethyl acetate: petroleum ether = 1:3) to obtain compound 11, 27.7 g of a colorless oil, with a yield of 86%.
[0044] Step 2 Synthesis of (3S)-hydroxybutyrate ethyl 8b
[0045] Calcium carbonate (4.3 g, 43.0 mmol) was added to water (108 mL) and stirred to form a suspension. Then the above solution was heated to 80° C., compound 11 (30.13 g, 143.3 mmol) was added dropwise at this temperature, and stirring was cont...
Embodiment 2
[0067] Step 1 (2R, 3R)-2-Methyl-3-hydroxybutyrate 9a
[0068] Under an ice bath, n-butyllithium (2.5M, 9.4 mL) was added dropwise to a solution of diisopropylamine (3.2 mL, 23.4 mmol) in tetrahydrofuran (6 mL). The mixture was cooled to -60°C, and a solution of 8a (1.40 g, 10.6 mmol) in tetrahydrofuran (2 mL) was added dropwise for 15 minutes. After 15 minutes, a mixed solution of methyl iodide (1.2 mL, 25.7 mmol) and hexamethylphosphoramide (1.5 mL) was added dropwise. After the mixture was stirred for 1 h, it was quenched with frozen saturated ammonium chloride (1.0 mL) and water (10 mL), and extracted with ethyl acetate (3×8 mL). The combined organic phase was washed sequentially with saturated brine (2 mL), dried over anhydrous sodium sulfate, filtered, concentrated under reduced pressure, and subjected to flash column chromatography (ethyl acetate: petroleum ether = 1:2) to obtain 1.13 g of a colorless oil Compound 9a, the yield was 72%.
[0069] Step 2 (2S, 3R)-2-methyl-3-...
Embodiment 3
[0088] Step 1 Synthesis of (3R)-Methanesulfonate Butyrate Ethyl 11
[0089] The synthesis method of (3R)-ethyl methanesulfonate butyrate 11 is the same as in Example 1.
[0090] Step 2 Synthesis of (3S)-hydroxybutyrate ethyl 8b
[0091] The synthesis method of (3S)-hydroxybutyrate ethyl 8b is the same as in Example 1.
[0092] Step 3 (2S, 3S)-2-methyl-3-hydroxybutyric acid ethyl ester 9b
[0093] The synthesis method of (2S, 3S)-2-methyl-3-hydroxybutyrate 9b is the same as in Example 1.
[0094] Step 4 (2R, 3S)-2-methyl-3-tetrahydropyranyl-1-butanol 10b
[0095] The synthesis method of (2R, 3S)-2-methyl-3-tetrahydropyranyl-1-butanol 10b is the same as in Example 1.
[0096] Step 5 (2R, 3S)-2-methyl-3-tetrahydropyranyl-1-p-toluenesulfonyloxybutane 6d
[0097] The synthesis method of (2R, 3S)-2-methyl-3-tetrahydropyranyl-1-p-toluenesulfonyloxybutane 6d is the same as in Example 1.
[0098] Step 6 (R)-1,3-Butanediol 12a
[0099] The synthesis method of (R)-1,3-butanediol 12a is the same as in Ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com