Zinc alloy electroplating baths and processes
A zinc-nickel alloy and polymer technology, applied in the field of electrodeposited zinc-nickel alloy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
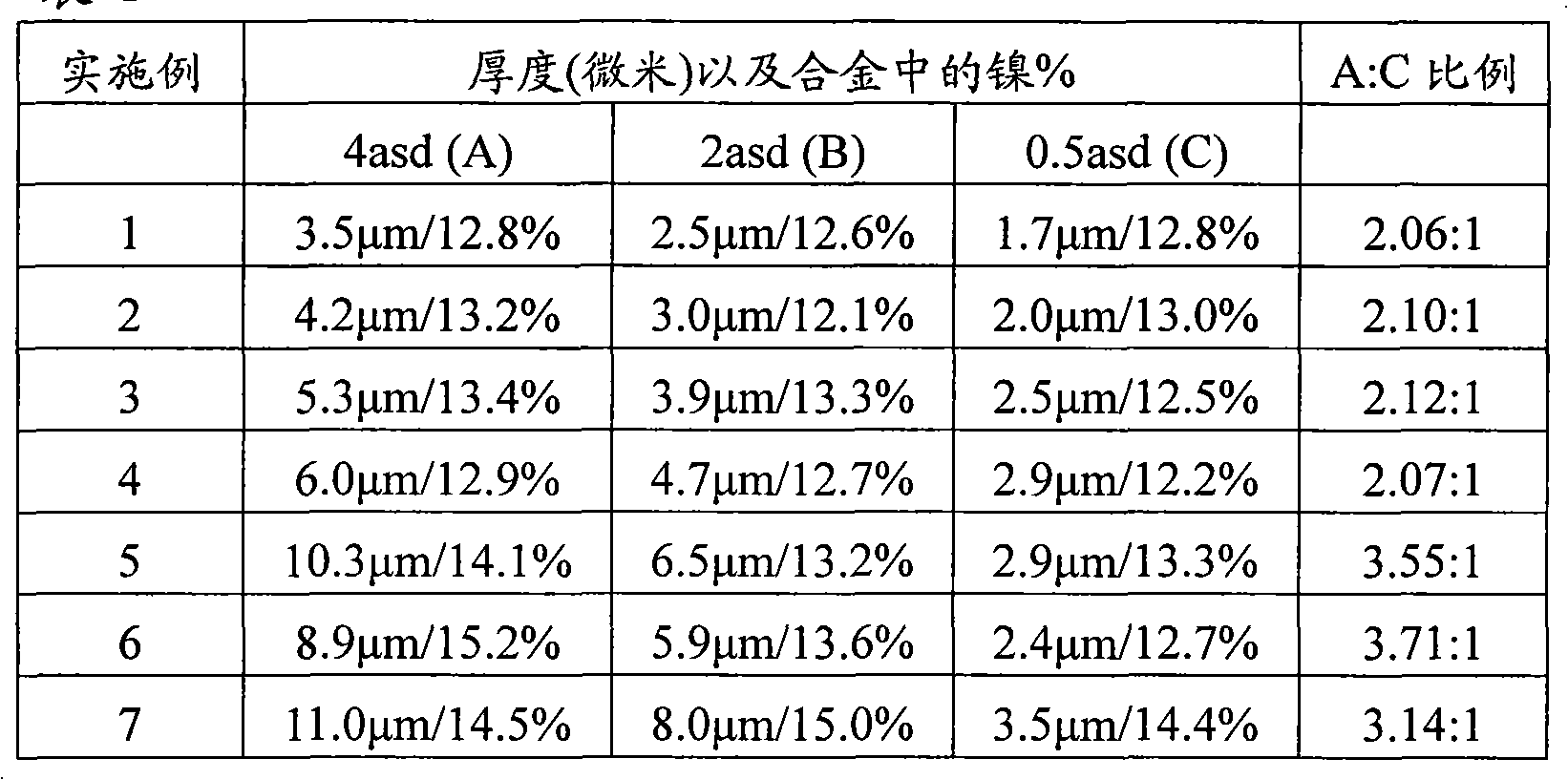
[0030] Prepare a kind of aqueous electrolytic bath that is suitable for plating zinc-nickel alloy, it contains the sodium hydroxide of 90g / L, the zinc ion of 8g / L, the nickel ion of 4g / L, the triethanolamine of 68g / L, the triethanolamine of 30g / L N,N,N',N'-tetrahydroxyisopropylethylenediamine, 12.5g / L of sodium silicate and 400mg / L of N,N'-bis[3-(dimethylamino)propyl] Polymer of urea and 1,1'-oxybis[2-chloroethane]. A bright steel Hull cell plate was plated at a current of 1 A in a Hull cell using a nickel anode for 20 minutes at a temperature of 30°C. The appearance of the plated plate is uniform and bright, with no visible defects. The deposit thicknesses and nickel alloy contents listed in Table 1 below were measured using a Fischerscope X-ray system XDL-B under conditions of current densities of 4A, 2A, and 0.5A per square decimeter as a whole of the plated plate.
Embodiment 2
[0032] Prepare a kind of aqueous electrolytic bath that is suitable for plating zinc-nickel alloy, it contains the sodium hydroxide of 90g / L, the zinc ion of 8g / L, the nickel ion of 4g / L, the triethanolamine of 68g / L, the triethanolamine of 30g / L N,N,N',N'-tetrahydroxyisopropylethylenediamine, 12.5g / L of sodium silicate and 100mg / L of N,N'-bis[3-(dimethylamino)propyl] N-[2-hydroxy- Polymers of 3-(2-propenyloxy)propyl] derivatives. A bright steel Hull cell plate was plated at a current of 1 A in a Hull cell using a nickel anode for 30 minutes at a temperature of 30°C. The appearance of the plated plate is uniform and bright, with no visible defects. The deposit thicknesses and nickel alloy contents listed in Table 1 below were measured using a Fischerscope X-ray system XDL-B under conditions of current densities of 4A, 2A, and 0.5A per square decimeter as a whole of the plated plate.
Embodiment 3
[0034] Prepare a kind of aqueous electrolytic bath that is suitable for plating zinc-nickel alloy, it contains the potassium hydroxide of 120g / L, the zinc ion of 8g / L, the nickel ion of 4g / L, the triethanolamine of 68g / L, the triethanolamine of 30g / L N,N,N',N'-tetrahydroxyisopropylethylenediamine, 12.5g / L of sodium silicate and 100mg / L of N,N'-bis[3-(dimethylamino)propyl] N-[2-hydroxy- Polymers of 3-(2-propenyloxy)propyl] derivatives. A bright steel Hull cell plate was plated at a current of 1 A in a Hull cell using a nickel anode for 30 minutes at a temperature of 30°C. The appearance of the plated plate is uniform and bright, with no visible defects. The deposit thicknesses and nickel alloy contents listed in Table 1 below were measured using a Fischerscope X-ray system XDL-B under conditions of current densities of 4A, 2A, and 0.5A per square decimeter as a whole of the plated plate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com