Whole blood INF-gamma specific antigen protein and preparation method and application thereof
An INF-, antigen protein technology, applied in microorganism-based methods, biochemical equipment and methods, chemical instruments and methods, etc., can solve the problems of high production cost, loss of protein activity, small molecular weight, etc., and achieve diagnosis in a short time. , high specificity, rapid diagnosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The synthesis of the target gene of embodiment 1ESAT6-CFP10 fusion protein and the construction of expression vector
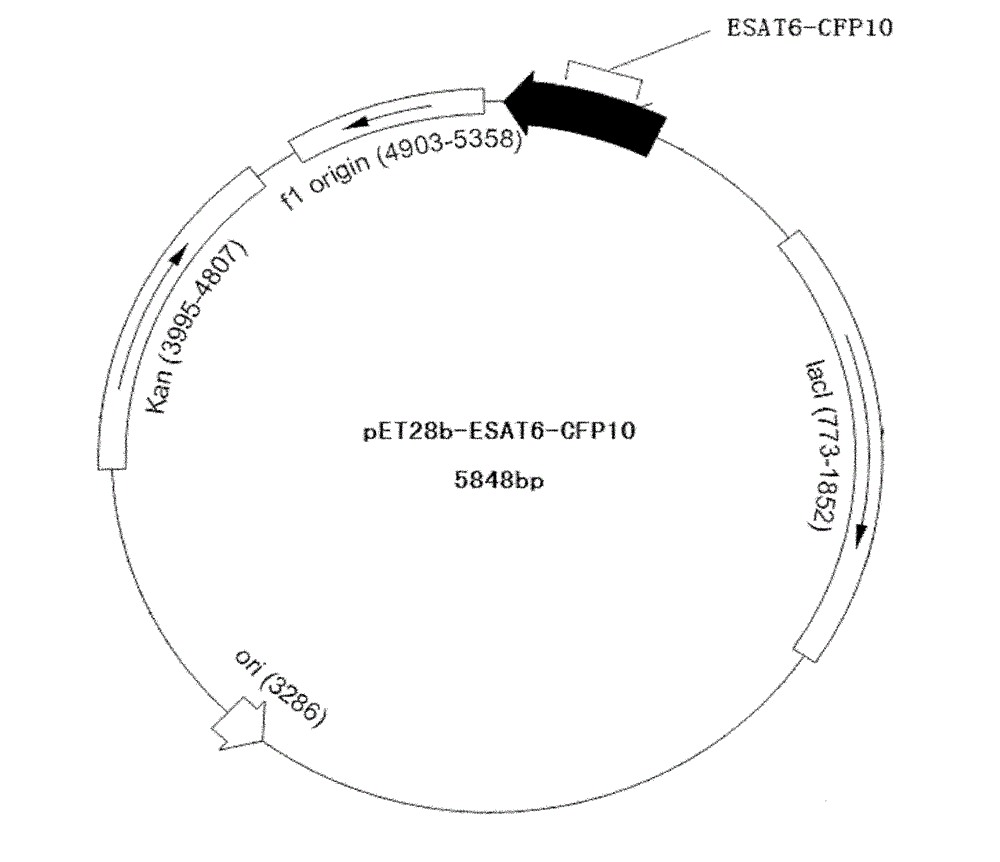
[0042] The DNA sequence of the artificially synthesized ESAT6-CFP10 was stored in DH5α, and then the DH5α and pET28b expression vectors were digested with NcoI and XhoI endonucleases respectively, and the target fragment and expression vector were recovered. 4 Under the action of DNA ligase, ligate at 4°C for 16 hours, transform into Escherichia coli BL21(DE3) by heat shock method, and culture at 37°C overnight. Pick colonies that grow well on the plate and have typical Escherichia coli characteristics and cultivate them in liquid LB medium. At the same time, add kanamycin with a final concentration of 30 μg / ml to the medium, and cultivate for 3 hours at 37°C and 220rpm , Extract the plasmid for enzyme digestion and sequencing identification, the enzyme map and sequencing map are in line with expectations. Vector construction diagram see figure 1 .
Embodiment 2
[0043] Example 2 Tandem expression of recombinant protein ESAT6-CFP10 Highly expressed in Escherichia coli
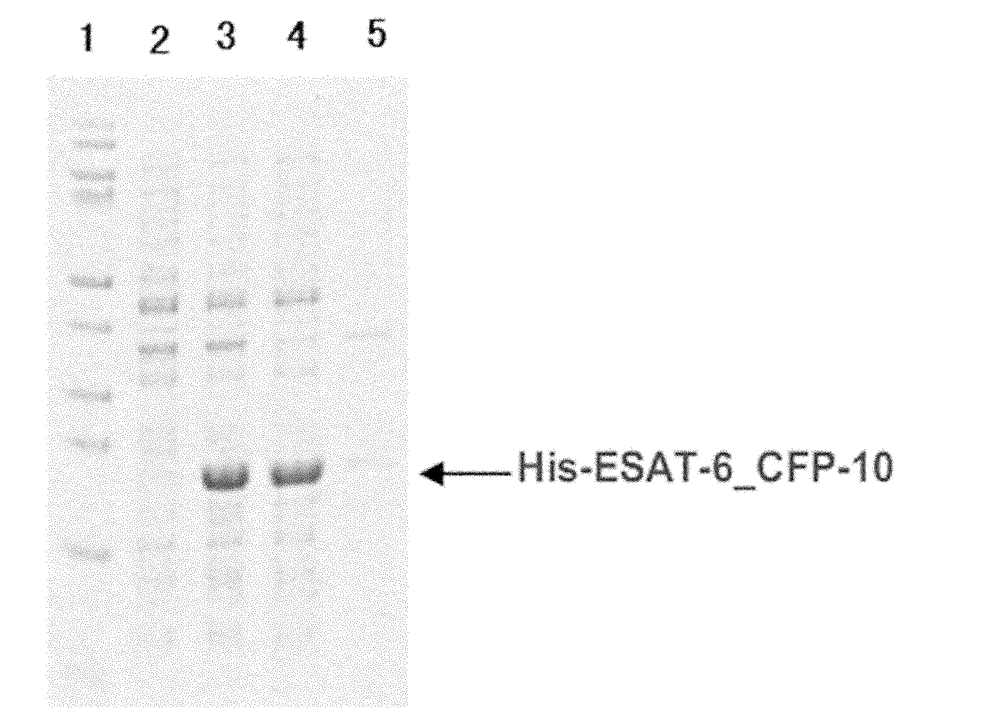
[0044] Pick colonies that grow well on the plate and have typical Escherichia coli characteristics and culture them in liquid LB medium, add ampicillin with a final concentration of 50 μg / ml and culture for 6 hours, add IPTG with a final concentration of 0.5 mM, 37 ° C, 200rpm, induced expression for 8 hours, collected bacteria, SDS-PAGE electrophoresis identification results see figure 2 .
Embodiment 3
[0045] Example 3 Purification of Recombinant Protein ESAT6-CFP10 Series
[0046] Protein purification was carried out according to Ni-NTA standard operating procedures. The brief steps are as follows. Take 50ml of the bacterial liquid and centrifuge to collect the engineered bacteria that induce expression, use ultrasonic waves to break the cells of the large intestine, centrifuge to get the supernatant, and the supernatant is in Tris (50mM, pH8.0) Dialyze in the buffer overnight, centrifuge to take the supernatant, directly load the sample on Ni-NTA resin equilibrated with Tris (50mM, pH8.0) buffer, elute with Tris (50mM, pH8.0) buffer after equilibration , and then eluted with 0-1M imidazole gradient, collected the eluted peaks, combined the eluted peaks, dialyzed overnight in Tris (50mM, pH8.0) buffer solution, and lyophilized the dialysate in a vacuum freeze dryer to obtain ESAT6-CFP10 freeze-dried powder, stored at -20--40°C for future use. SDS-PAGE identification of pur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com