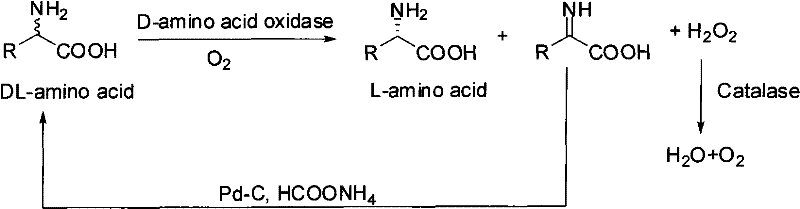
Bio-catalytic deracemization preparation method of non-natural L-amino acid
A technology of biocatalysis and biocatalyst, which is applied in the field of preparation of chiral pharmaceutical intermediates by biocatalytic asymmetric oxidation, can solve the problems of sensitivity, easy decomposition, unreported non-natural L-amino acid method, etc., and achieve simple process and environmental friendliness , good versatility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0021] Example 1: L-2-aminobutyric acid
[0022] 2.06g (0.02mol) DL-2-aminobutyric acid is dissolved in 50ml of ammonium formate (1.0mol / L, adjust the pH to 7.2 with ammonia water) solution, add 2.06g immobilized D-amino acid oxidase (58U / g), 1ml of catalase solution (50000U / ml), 1g of Pd-C catalyst (10%), oxygen is continuously introduced, and the reaction is stirred at 30°C for 16h, the optical rotation of the reaction solution no longer increases. Filter to remove immobilized D-amino acid oxidase and Pd-C, concentrate the filtrate to about 10ml, adjust the isoelectric point, freeze to precipitate white crystals, and dry to obtain 1.8g L-2-aminobutyric acid, the yield is 87%, ee>99 %.
Example Embodiment
[0023] Example 2: L-2-aminovaleric acid
[0024] 2.90g (0.025mol) DL-2-aminovaleric acid is dissolved in 50ml of ammonium formate (1.0mol / L, adjust the pH to 7.2 with ammonia) solution, add 2.90g immobilized D-amino acid oxidase (58U / g), 1.5ml of catalase solution (50000U / ml), 1g of Pd-C catalyst (10%), oxygen was continuously introduced, and the reaction was stirred at 30°C for 16h, and the optical rotation of the reaction solution no longer increased. The immobilized D-amino acid oxidase and Pd-C were removed by filtration, the filtrate was concentrated to about 10ml, the isoelectric point was adjusted, white crystals were precipitated by freezing, and dried to obtain 2.6g L-2-aminovaleric acid, the yield was 90%, ee>99 %.
Example Embodiment
[0025] Example 3: L-2-Aminoadipic acid
[0026] 3.22g (0.02mol) DL-2-aminoadipate was dissolved in 50ml ammonium formate (1.0mol / L, adjust the pH to 7.2 with ammonia) solution, and 3.22g immobilized D-amino acid oxidase (58U / g) was added , 1ml catalase solution (50000U / ml), 1g Pd-C catalyst (10%), oxygen is continuously introduced, and the reaction is stirred at 30°C for 20h, the optical rotation of the reaction solution no longer increases. The immobilized D-amino acid oxidase and Pd-C were removed by filtration, the filtrate was concentrated to about 10ml, the isoelectric point was adjusted, white crystals were precipitated by freezing, and dried to obtain 2.8g L-2-aminoadipate, the yield was 87%, ee> 99%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap