Cancertestis antigen HCA587 protein vaccine and application thereof
A technology of protein vaccine and testicular antigen, which is applied in the field of medical oncology, can solve the problems of tumor growth rate and survival period of tumor-bearing mice that have not seen the anti-tumor effect of epitope peptide vaccine, so as to inhibit the growth rate, prolong the survival period, and achieve good results. The effect of anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Expression and purification of embodiment 1HCA587 recombinant protein
[0042] The cDNA encoding HCA587 (AF239802) was ligated to the pGEX-6p-1 vector through restriction sites BamHI and Sal I, transformed into Escherichia coli BL21(DE3), induced expression with 0.2mM IPTG at 25°C for 5 hours, and centrifuged at 4000rpm at 4°C for 15 minutes , collect the bacteria, dissolve in the lysate (pH 7.4PBS, 1mM EDTA and 1mM DTT), sonicate 10 times, each lasting 15 seconds, centrifuge at 14,000rpm for 15 minutes, and discard the precipitate.
[0043] The fusion protein was firstly subjected to affinity chromatography on a GST Sepharose 4B (GE) column, washed and dissolved in lysis buffer (20mM Tris, 150mM NaCl, 1mM DTT, 1mM EDTA, pH7.4), and PPase (250ug / 1ml resin ) at 4°C for 3 hours to remove GST. The collected protein was passed through HiTrap Q HP (GE) column, the buffer solution was 20mM Hepes, 1M NaCl, pH6.7, and the flow rate was 5ml / min. The collected fractions were th...
Embodiment 2
[0045] Preparation and immunization procedure of embodiment 2HCA587 protein vaccine
[0046] Option One
[0047] Mix and emulsify 50 μl (10 μg) of HCA587 protein and 50 μl (12 μg) of CpG to make a protein vaccine.
[0048] Take 6-8 week-old mice (C57BL / 6), 3 mice in each group, and inject 100 μl of the above-mentioned protein vaccine into each mouse, subcutaneously inject at the base of the tail for the first immunization, and 20 days later, inject again for the second immunization.
[0049] Option II
[0050] Mix and emulsify 50 μl (10 μg) of HCA587 protein and 50 μl (12 μg) of ISCOM to make a protein vaccine.
[0051] Take 6-8 week-old mice (C57BL / 6), 3 mice in each group, and inject 100 μl of the above-mentioned protein vaccine into each mouse, subcutaneously inject at the base of the tail for the first immunization, and 20 days later, inject again for the second immunization.
[0052] third solution
[0053] Mix and emulsify 50 μl (10 μg) of HCA587 protein, 25 μl (12 μ...
Embodiment 3
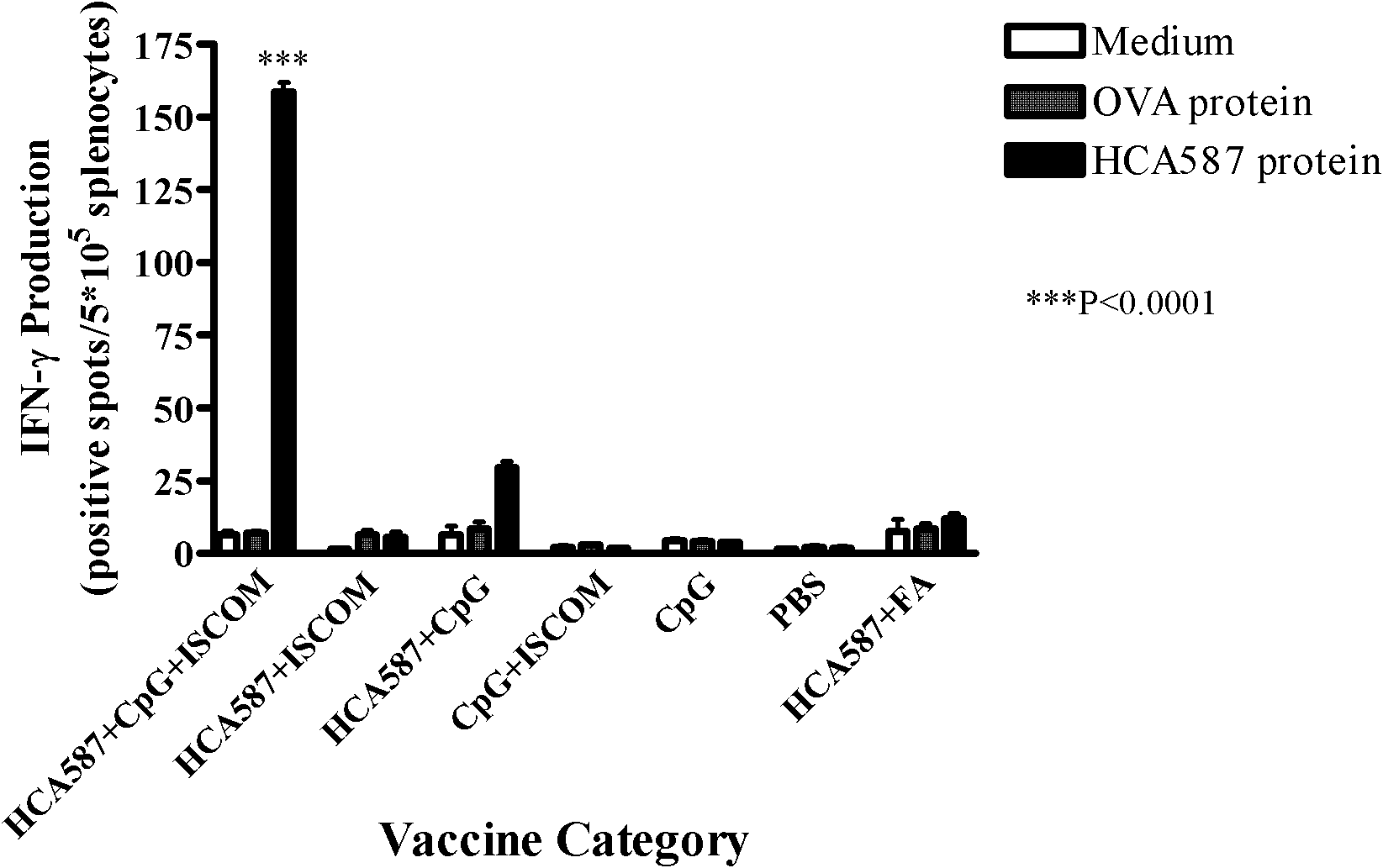
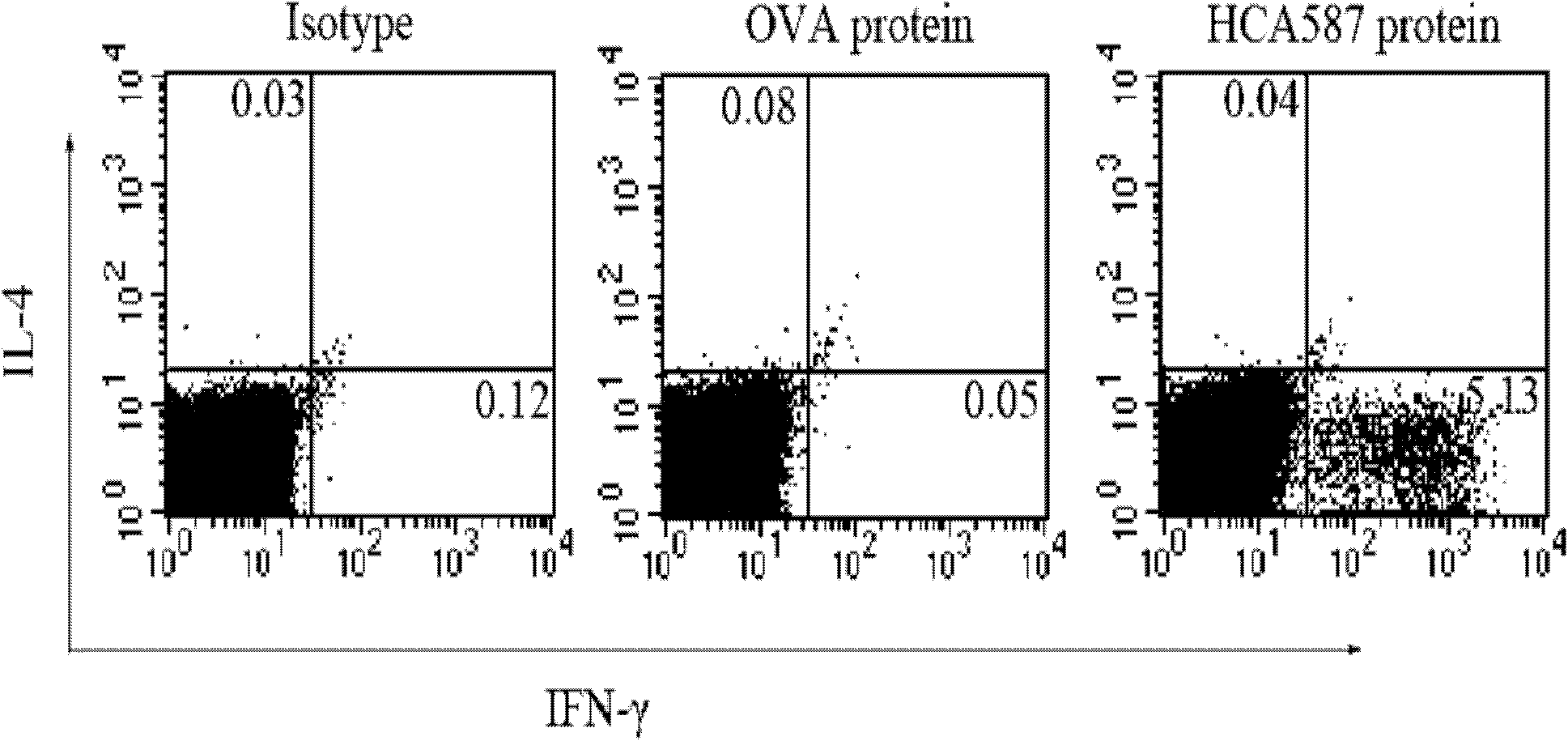
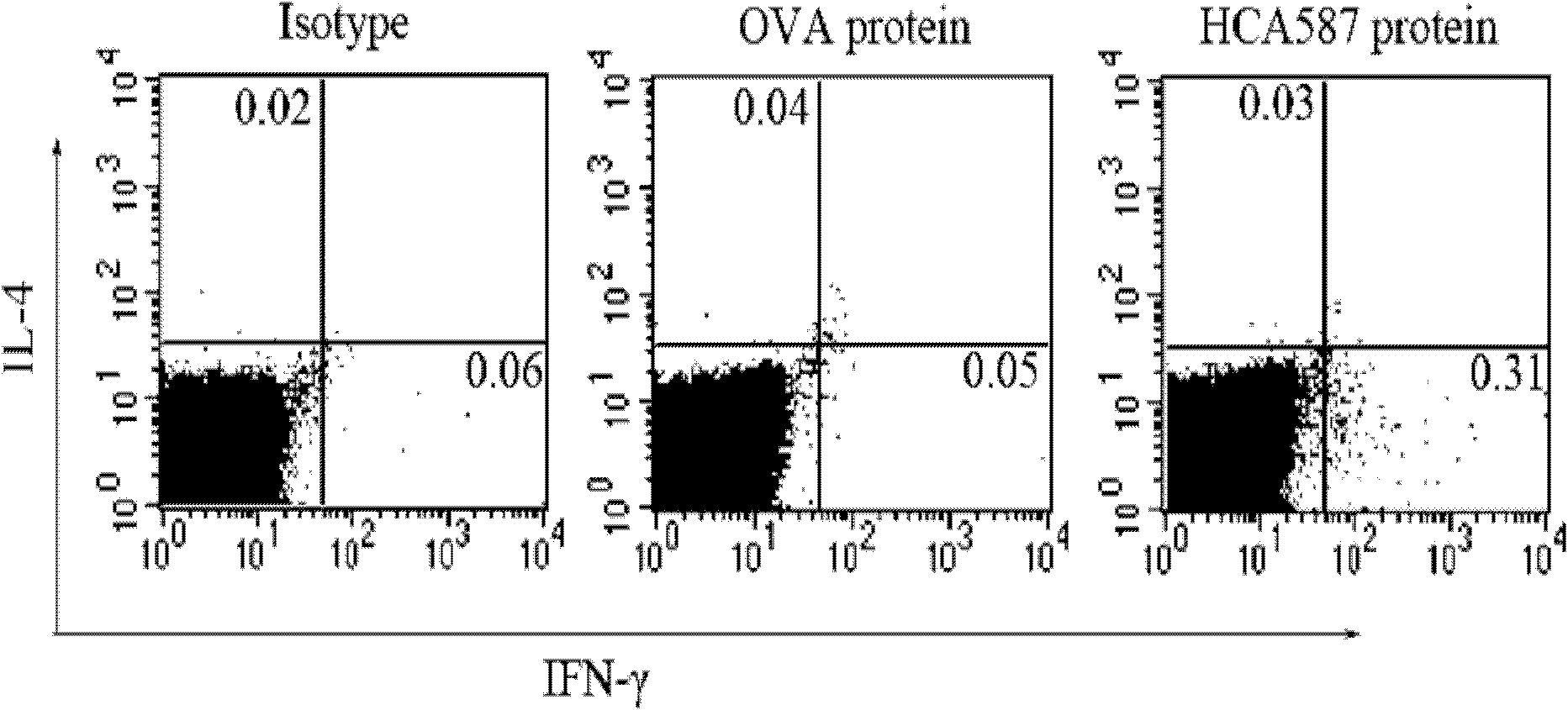
[0060] Example 3 Enzyme-linked immunospot (ELISpot) method to measure the cell frequency of secreting IFN-γ in mouse splenocytes
[0061] (1) 96-well flat-bottomed nitrocellulose plates were coated with anti-mouse IFN-γ monoclonal antibody (5 μg / ml in pH 9.6 carbonate buffer), and left overnight at 4°C;
[0062] (2) After washing with 0.05% Tween PBS (PBST) the next day, the plate was sealed with 1640 culture solution containing 10% FCS in a 37°C incubator for 2 hours;
[0063] (3) After washing with PBST, for the immunized mice described in Example 2, add 5×10 5 Splenocytes, then add 2.5μg / ml HCA587 protein, the control group includes media control and OVA irrelevant protein control, each triplicate hole, at 37 ° C, 5% CO 2 Incubate for 20 hours;
[0064] (4) Wash the plate 3 times with PBST to remove residual cells; then add biotin-labeled anti-mouse IFN-mAb (1.5 μg / ml), incubate at 37°C for 2 hours, and wash the plate 6 times;
[0065] (5) Add alkaline phosphatase-labele...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com