Ophthalmic gel composition
A technology of ophthalmic gel and composition, which is applied in the field of ophthalmic gel composition and its preparation, and can solve problems such as difficulty in absorption, loss, foreign body sensation in the eyes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
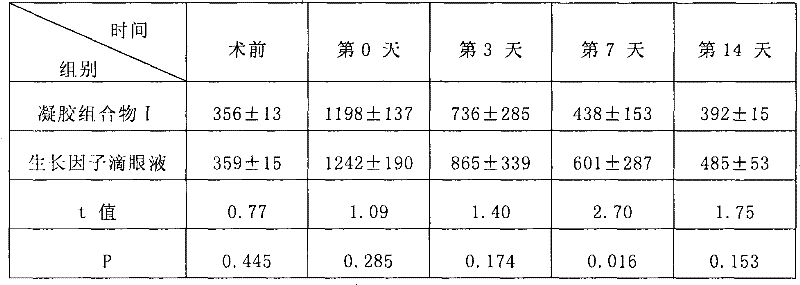
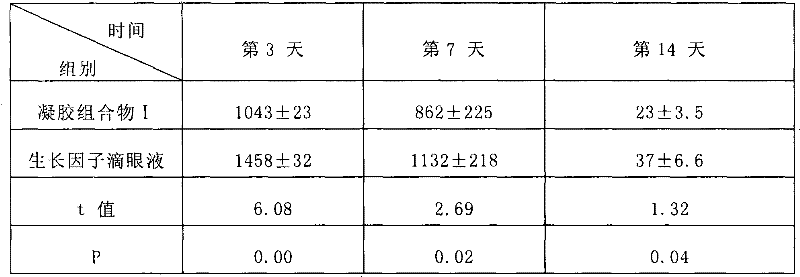
Examples
Embodiment 1
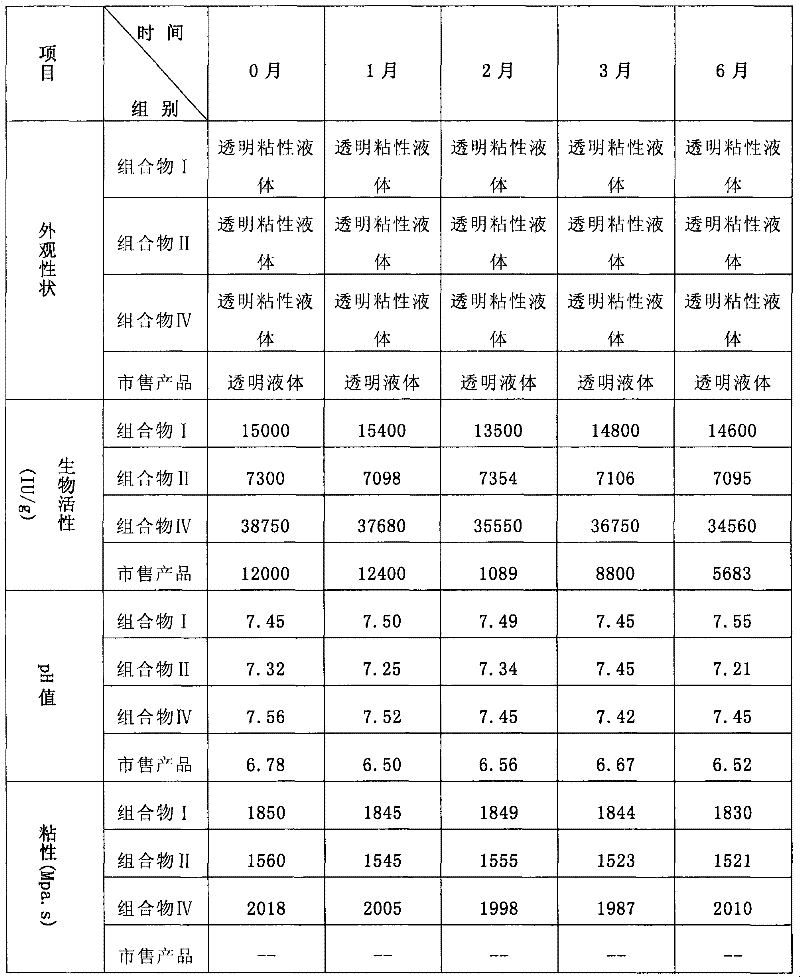
[0054] Preparation of Ophthalmic Gel Composition I
[0055] The components in the prescription are shown in the table below
[0056] component name
% of total
role in prescription
recombinant human epidermal growth factor
1×10 7 IU
0.002%
Main drug
sodium hyaluronate
1.5g
0.15%
Main drug
1.0g
0.1%
stabilizer
3.0g
0.3%
Nutrients
[0057] Glycine
3.0g
0.3%
Nutrients
3.0g
0.3%
Nutrients
8.5g
0.85%
isotonicity regulator
Sodium dihydrogen phosphate monohydrate
1.7g
0.17%
pH regulator
Water for Injection
978.28g
97.828%
[0058] Preparation:
[0059] 1. In a 100-grade purification environment, weigh 1.5g of sodium hyaluronate and 1.0g of bovine seru...
Embodiment 2
[0064] Preparation of Ophthalmic Gel Composition II
[0065] The components in the prescription are shown in the table below
[0066] component name
% of total
role in prescription
recombinant human epidermal growth factor
5×10 6 IU
0.001%
Main drug
1.0g
0.1%
Main drug
1.0g
0.1%
stabilizer
3.0g
0.3%
Nutrients
3.0g
0.3%
Nutrients
8.5g
0.85%
isotonicity regulator
2.2g
0.22%
pH regulator
0.2g
0.02%
pH regulator
Water for Injection
981.09g
98.109%
[0067] Preparation:
[0068] 1. In a 100-grade purification environment, weigh 1.0g of sodium hyaluronate and 1.0g of bovine serum albumin in a 10...
Embodiment 3
[0073] Preparation of Ophthalmic Gel Composition III
[0074] The components in the prescription are shown in the table below
[0075] component name
% of total
role in prescription
recombinant human epidermal growth factor
1×10 6 IU
0.0002%
Main drug
0.5g
0.05%
Main drug
bovine serum albumin
1.0g
0.1%
stabilizer
2.2
0.22%
pH regulator
0.2g
0.02%
pH regulator
Sodium chloride
8.5g
0.85%
isotonicity regulator
Water for Injection
987.58g
98.758%
[0076] Preparation:
[0077] 1. In a class 100 purification environment, weigh 0.5g of sodium hyaluronate and 1.0g of bovine serum albumin into a 1000ml volumetric flask according to the prescription, add about 300ml of water for injection an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com