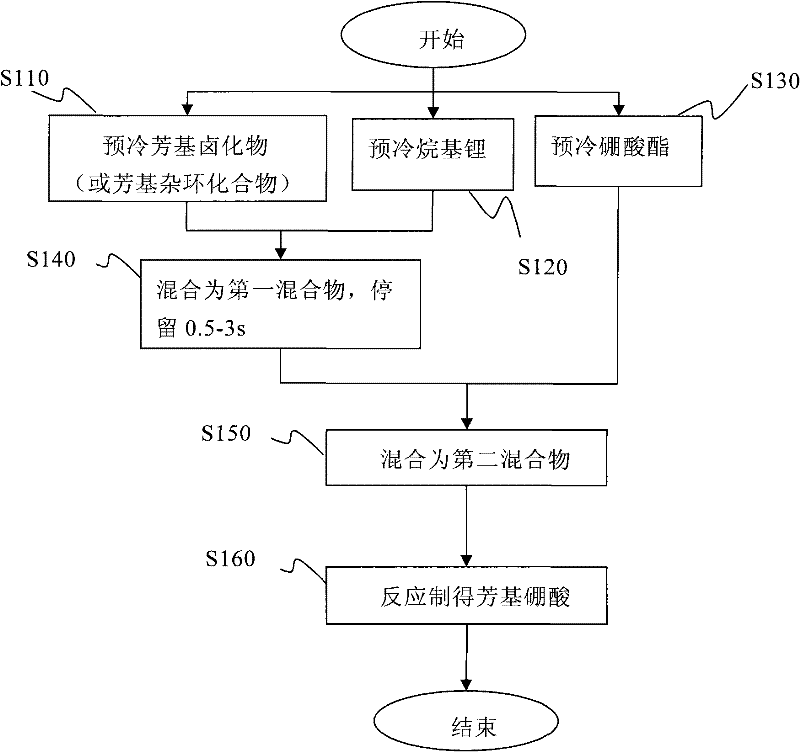
Method and apparatus for continuously producing aryl boric acid
An aryl boronic acid and aryl technology, which is applied in the field of continuous preparation of organic boronic acids, can solve the problems of difficulty in improving reaction yield and production efficiency, and achieve the effects of shortening residence time, continuous production and improving reaction yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Synthesis of 3-thiophene boronic acid
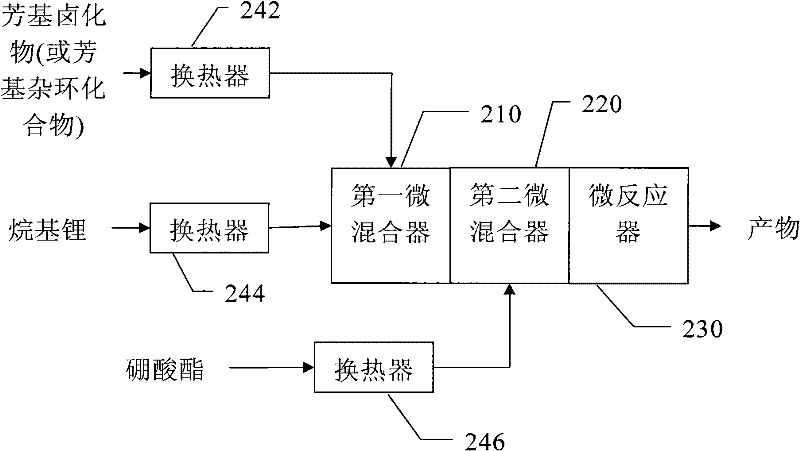
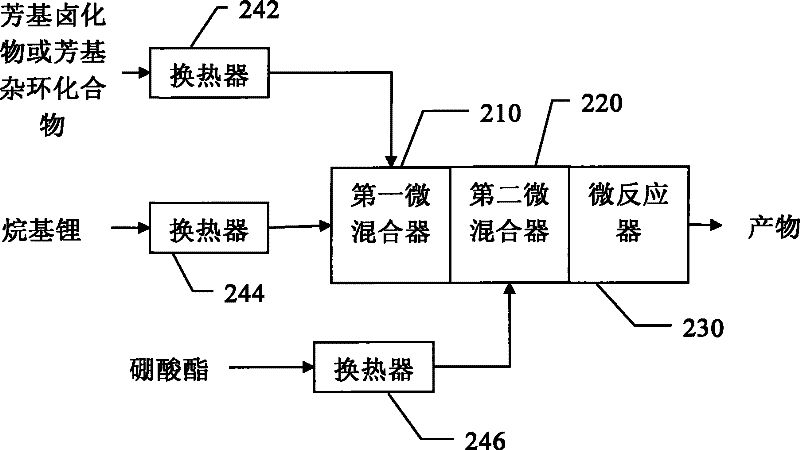
[0037] Pass the 3-bromothiophene / tetrahydrofuran solution with a flow rate of 9mL / min and a molar concentration of 0.75mol / L and a butyllithium / n-hexane solution with a flow rate of 3mL / min and a molar concentration of 2.7mol / L through the heat exchanger. 242 and 244 are pre-cooled to -50°C and injected into the first micromixer 10 to mix. Subsequently, it is mixed with the n-butyl borate / tetrahydrofuran solution with a flow rate of 9 mL / min and a molar concentration of 0.9 mol / L, which is pre-cooled to -50°C by the heat exchanger 246, through the second micro mixer 220, and then enters the micro reactor The reaction was carried out at 230, and the reaction temperature was controlled to -45°C. The residence volume of the mixture of 3-bromothiophene and butyllithium is 530 μL, including the inside of the first micromixer 210, the outlet of the first micromixer 210 and the inlet of the second micromixer 220, and the residence time is ...
Embodiment 2
[0043] Synthesis of 5-pyrimidine boronic acid
[0044] Pass the 5-bromopyrimidine / tetrahydrofuran solution with a flow rate of 27mL / min and a molar concentration of 0.25mol / L and a butyllithium / n-hexane solution with a flow rate of 6mL / min and a molar concentration of 2.7mol / L through the heat exchanger. 242 and 244 are pre-cooled to -65°C and injected into the first micromixer 210 to mix. Subsequently, it is mixed with the n-butyl borate / tetrahydrofuran solution with a flow rate of 11 mL / min and a molar concentration of 0.6 mol / L, which is precooled to -55°C by the heat exchanger 246, through the second micromixer 220, and then enters the microreactor The reaction was carried out at 230, and the reaction temperature was controlled to -50°C. The residence volume of the mixture of 5-bromopyrimidine and butyllithium is 440 μL, including the inside of the first micromixer 210, the outlet of the first micromixer 210 and the inlet of the second micromixer 220, and the residence time ...
Embodiment 3
[0047] Synthesis of 4-cyanophenylboronic acid
[0048] The 4-bromobenzonitrile / tetrahydrofuran solution with a flow rate of 11 mL / min and a molar concentration of 0.5 mol / L and a butyl lithium / n-hexane solution with a flow rate of 3 mL / min and a molar concentration of 2.2 mol / L were passed through heat exchange. The reactors 242 and 244 are pre-cooled to -65° C., and injected into the first micro mixer 210 for mixing. Subsequently, it is mixed with the n-butyl borate / tetrahydrofuran solution with a flow rate of 11 mL / min and a molar concentration of 0.6 mol / L, which is precooled to -55°C by the heat exchanger 246, through the second micromixer 220, and then enters the microreactor The reaction was carried out at 230, and the reaction temperature was controlled to -50°C. The residence volume of the mixture of 4-bromobenzonitrile and butyllithium is 440 μL, including the inside of the first micromixer 210, the outlet of the first micromixer 210, and the inlet of the second micromi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com