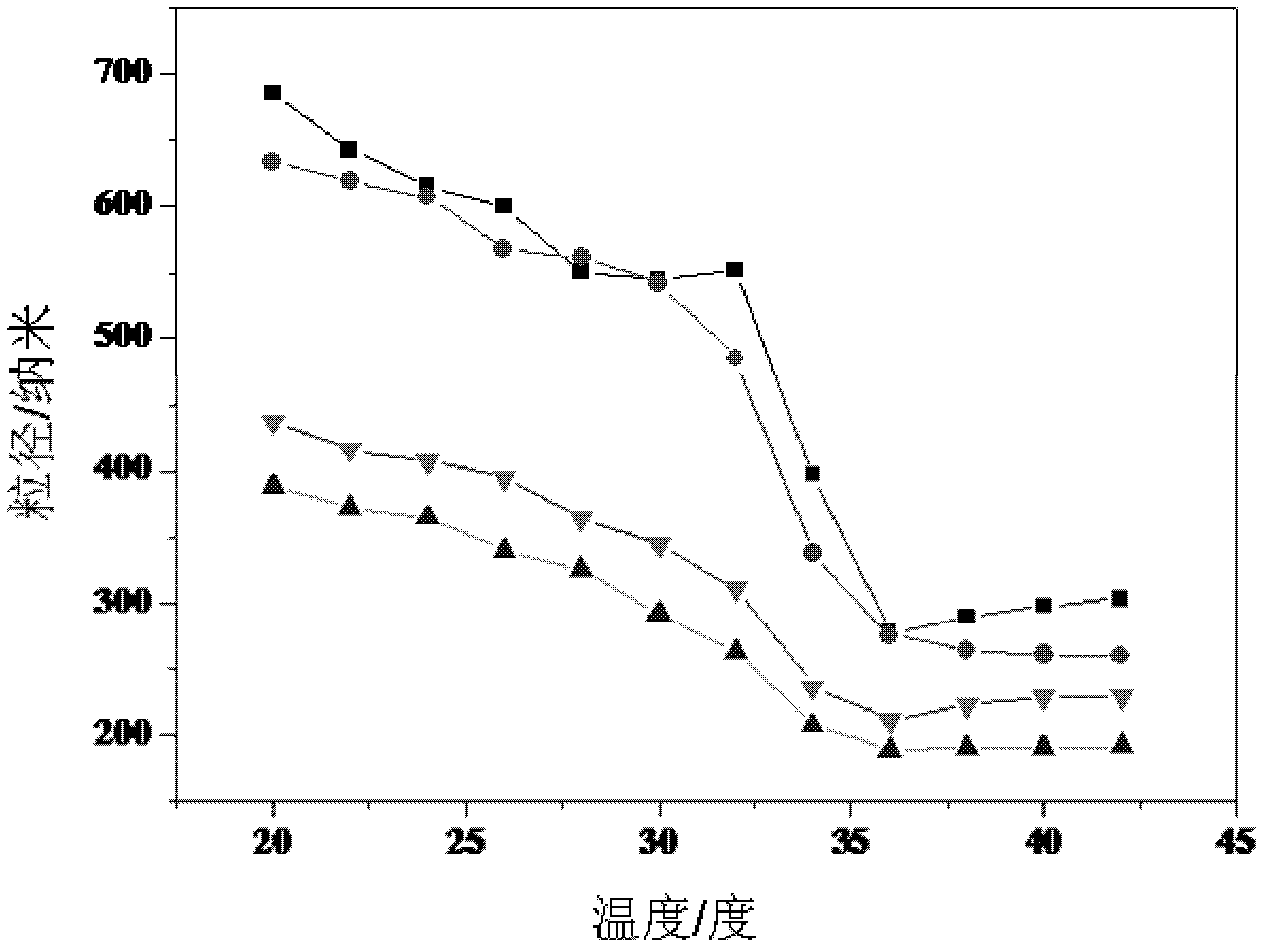
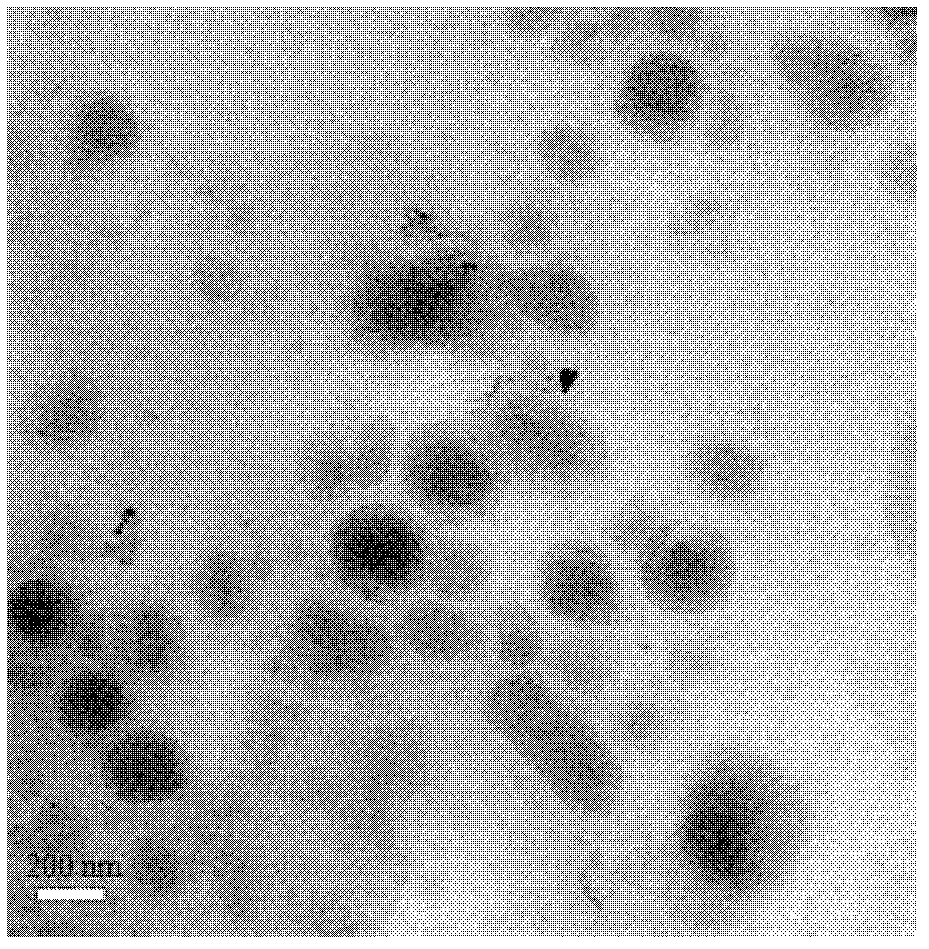Temperature-sensitive core-shell vesicle controlled-release drug carrier, preparation method and application thereof
A controlled-release drug and temperature-sensitive technology, which is applied in pharmaceutical formulations, liposome delivery, cosmetic preparations, etc., can solve the problems of not preparing a core-shell structure, short circulation period in the body, poor structural stability, etc., and achieve good Effects of rapid temperature response, good biocompatibility, and improved mechanical strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 1. Dissolve hyaluronic acid in ultrapure water at a concentration of 2 mg / mL, add triethylamine, tetrabutylammonium bromide and GMA (glycidyl methacrylate) at a weight ratio of 1:1:1, After reacting at room temperature for 10 hours, incubate at 40°C for 2 hours, freeze-dry for 2 days, and dialyze for 5 days.
[0044] 2. Add 1 mg rhodamine 6G to the phospholipid solution, transfer the solution into a round bottom flask, evaporate the solvent, and dry it in vacuum at room temperature for 2-4 hours to prepare a phospholipid film containing rhodamine 6G. After releasing the vacuum, put it into a 50-degree water bath to preheat for 20 minutes; add a buffer solution to hydrate and prepare liposomes wrapping rhodamine 6G, and make it pass through a dextran microgel column to separate the unencapsulated part. Liposomes encapsulated with Rhodamine 6G were obtained.
[0045] 3. Dilute the reaction solution obtained in step 1 with ultrapure water, add dropwise to the liposome sol...
Embodiment 2
[0052] 1. Dissolve chondroitin in ultrapure water at a concentration of 4 mg / mL, add 0.6 mL of triethylamine, 0.6 g of tetrabutylammonium bromide and 0.6 mL of GMA, react at room temperature for 20 hours, and incubate at 60°C for 2 hours. It was then freeze-dried for 5 days and dialyzed for 8 days.
[0053] 2. Add 0.5 mg rhodamine 6G to the phospholipid solution, and freeze-dry to prepare a lipid film containing rhodamine 6G. After adding buffer solution for hydration, the rhodamine 6G-encapsulated liposome is prepared, and the unencapsulated part is separated through a dextran microgel column to obtain the rhodamine 6G-encapsulated liposome.
[0054] 3. Dilute the reaction solution obtained in step 1 with ultrapure water, add dropwise to the liposome solution obtained in step 2, and sonicate for 30 minutes.
[0055] 4. Add 1 part of MBA (N, N-methylenebisacrylamide) and 10 parts of NIPAM (N-isopropylacrylamide) to the reaction solution obtained in step 3, and then add 1 part...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com