Method for preparing magnesium-doped xLiFePO4.yLi3V2(PO4)3 lithium ion battery anode material
A technology for lithium-ion batteries and cathode materials, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of unfriendly environment and poor rate performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
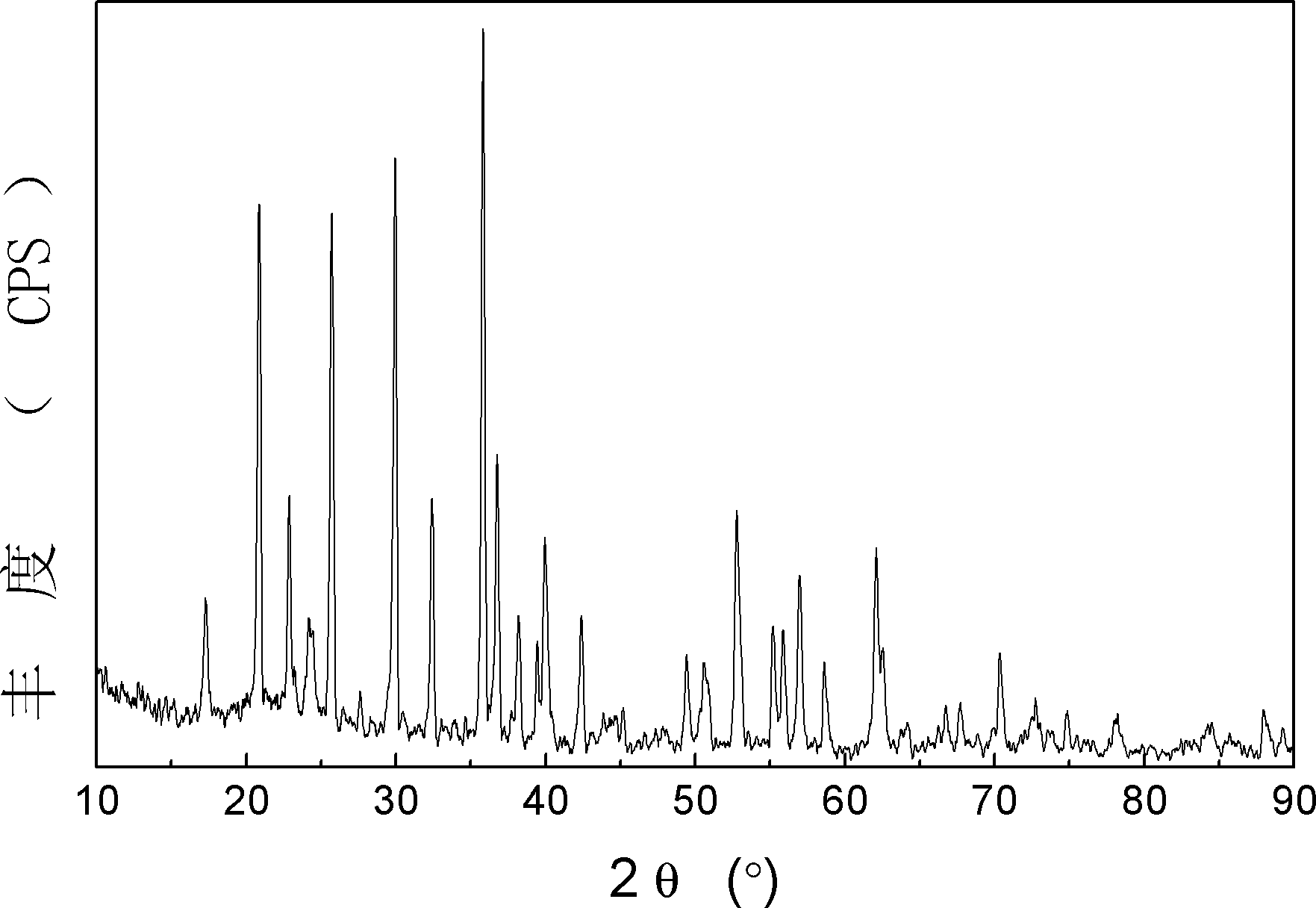
[0010] Specific implementation mode 1: In this implementation mode, magnesium doped xLiFePO 4 ·yLi 3 V 2 (PO 4 ) 3 The preparation method of lithium-ion battery cathode material is carried out according to the following steps: one, according to the molar ratio of Li element, Fe element, V element, Mg element, P element and C element is (x+3y)~1.1(x+ 3y): x: 2y: 0.1(x+y)~0.5(x+y):(x+3y):0.5(x+3y)~2(x+3y) Weigh lithium source, iron source, vanadium Salt, magnesium salt, phosphoric acid source and carbon source are mixed to obtain a mixture, and then the mixture is placed in a ball mill, and after adding a dispersant, it is wet-milled for 2-12 hours to obtain a mixture, wherein the volume ratio of the dispersant to the mixture is 1- 10:1, the dispersant is absolute ethanol, acetone or water, 0.05≤x / (x+y)≤0.95; 2. Pre-sinter the mixture obtained in step 1 under the protection of 250~450℃ and protective gas 2~6h; 3. Then the pre-sintered mixture is calcined at 570~870℃ and und...
specific Embodiment approach 2
[0012] Specific embodiment two: the difference between this embodiment and specific embodiment one is: the lithium source described in step one is LiOH·H 2 O, LiF, Li 2 CO 3 、LiCH 3 COO·H 2 O and LiNO 3 one or a combination of several of them. Other steps and parameters are the same as in the first embodiment.
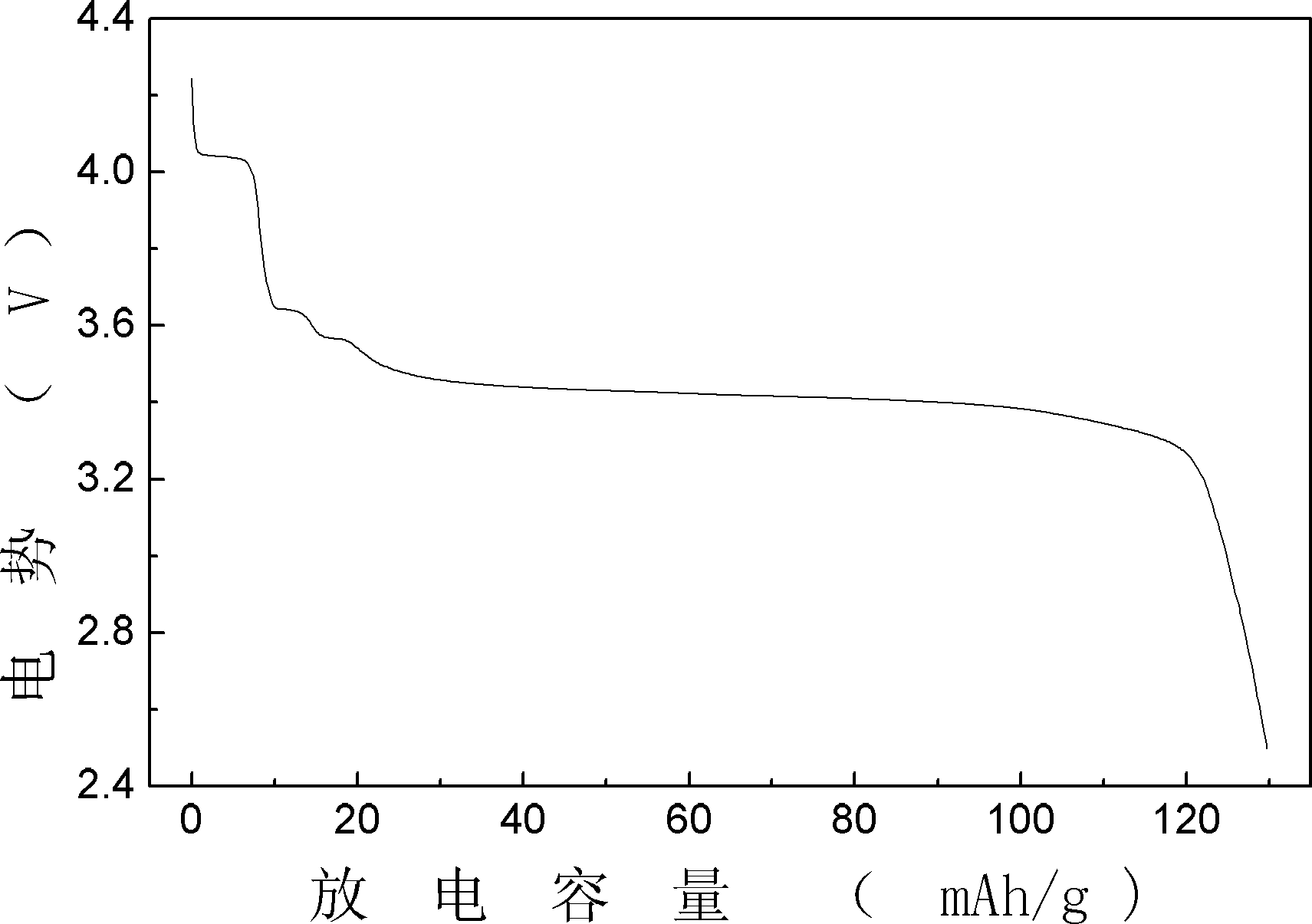
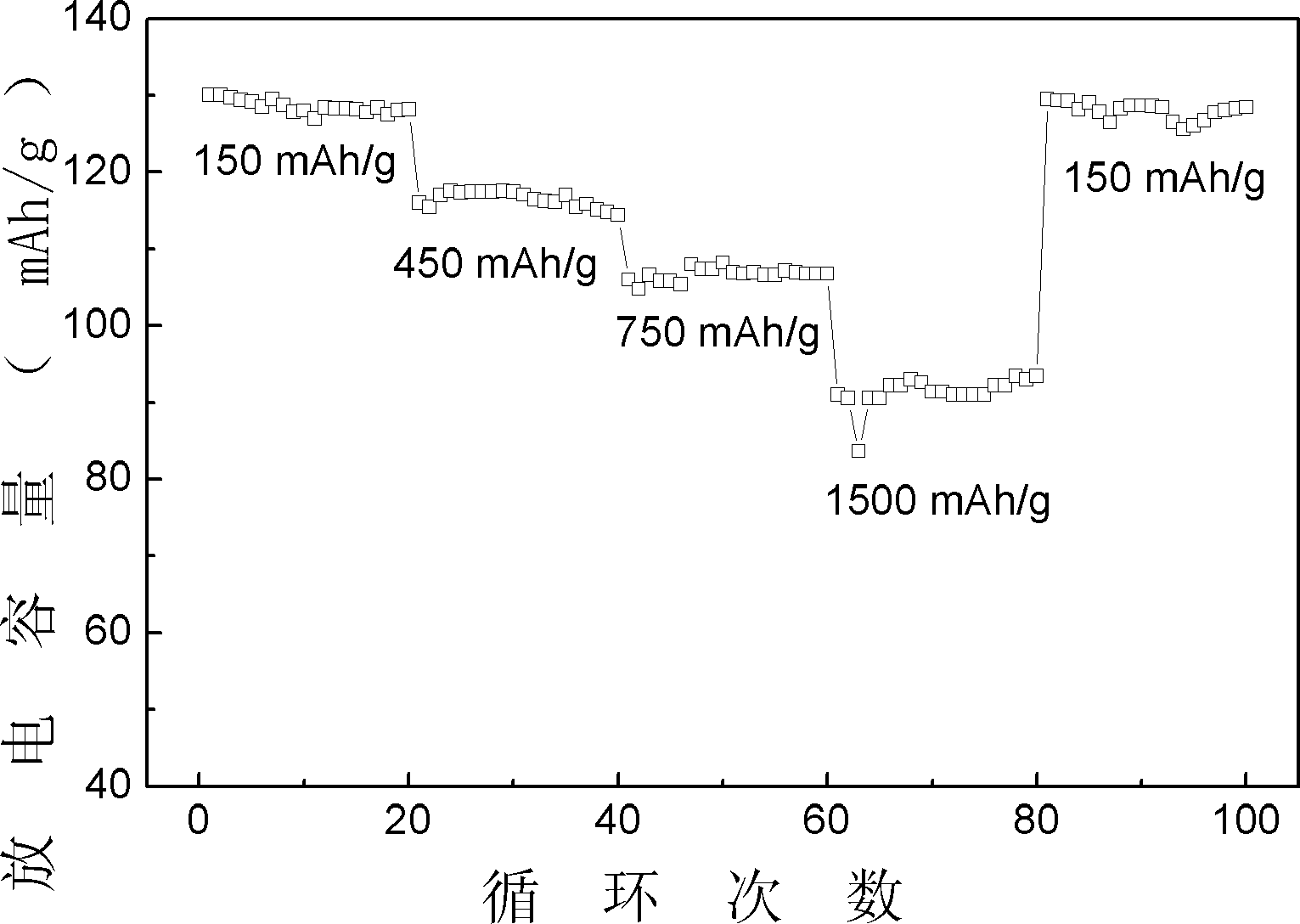
[0013] In this embodiment, when the lithium source is a mixture, various lithium sources are mixed in any ratio. Magnesium-doped xLiFePO prepared in this embodiment 4 ·yLi 3 V 2 (PO 4 ) 3 With higher first discharge specific capacity and capacity retention rate, the prepared Mg-doped xLiFePO 4 ·yLi 3 V 2 (PO 4 ) 3 The first discharge specific capacity of the electrode is 130mAh g -1 . After 400 cycles, the capacity retention rate of the former is not less than 95%. And found that this positive electrode has good rate discharge performance, when discharged at 10C, the discharge specific capacity is 90mAh g -1 , the capacity hardly fades after 20 cycle...
specific Embodiment approach 3
[0014] Embodiment 3: The difference between this embodiment and Embodiment 1 or 2 is that the iron source in Step 1 is ferrous oxalate, ferric phosphate, hydrated ferric phosphate, ferric nitrate or iron oxide. Other steps and parameters are the same as those in Embodiment 1 or Embodiment 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com