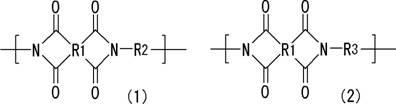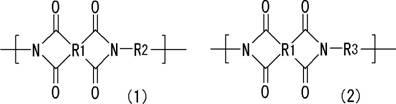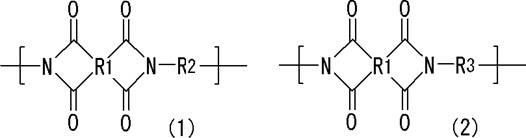Resin composition for printed wiring board
A printed circuit board and resin composition technology, which is applied in the direction of printed circuit, printed circuit manufacturing, printed circuit parts, etc., can solve unsatisfactory, unsatisfactory changes in elastic modulus, warping or cracking of printed circuit boards and other problems, to achieve the effect of small temperature change, excellent solvent resistance, excellent heat resistance and solvent resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0156] Add 25 parts by mass of 4,4'-(hexafluoroisopropylidene)-bis-(phthalic di Formic dianhydride) (hereinafter referred to as 6FDA), 69.9 parts by mass of γ-butyrolactone, 7 parts by mass of toluene, 55.7 parts by mass of diaminosiloxane X-22-9409 (manufactured by Shin-Etsu Chemical Co., Ltd.) (amine equivalent 665), 6.7 parts by mass of 2,6-bis(1-hydroxy-1-trifluoromethyl-2,2,2-trifluoroethyl)-1,5-naphthalenediamine (hereinafter referred to as HFA-NAP ), and stirred at 45°C for 2 hours under nitrogen flow to react. Next, the temperature of the reaction solution was raised, and water of condensation was azeotropically removed with toluene under a nitrogen stream while maintaining the temperature at about 160°C. When it was confirmed that a predetermined amount of water had accumulated in the moisture quantitative receiver and no outflow of water was observed, the temperature was further increased, and the mixture was stirred at 200° C. for 1 hour. Thereafter, cooling was t...
Synthetic example 2
[0159] In a 500mL detachable flask equipped with a moisture quantitative receiver connected to a reflux cooler, a nitrogen introduction tube, and a stirrer, add 32 parts by mass of 6FDA, 28.6 parts by mass of γ-butyrolactone, 28.6 parts by mass of Ipsole 150, and 7 parts by mass of toluene , 50.1 parts by mass of diaminosiloxane KF-8010 (manufactured by Shin-Etsu Chemical Co., Ltd.) (430 amine equivalents), and 6.3 parts by mass of HFA-NAP were stirred and reacted at 45° C. for 2 hours under a nitrogen stream. Next, the temperature of the reaction solution was raised, and water of condensation was azeotropically removed with toluene under a nitrogen stream while maintaining the temperature at about 160°C. When it was confirmed that a predetermined amount of water had accumulated in the moisture quantitative receiver and no outflow of water was observed, the temperature was further increased, and the mixture was stirred at 200° C. for 1 hour. Thereafter, cooling was terminated,...
Synthetic example 3
[0162] In a 500mL detachable flask equipped with a moisture quantitative receiver connected to a reflux cooler, a nitrogen introduction tube, and a stirrer, add 23 parts by mass of 3,3',4,4'-benzophenone tetracarboxylic dianhydride (hereinafter , called BTDA), 30.9 parts by mass of γ-butyrolactone, 30.9 parts by mass of Ipsole 150, 7 parts by mass of toluene, 49.0 parts by mass of diaminosiloxane KF-8010 (manufactured by Shin-Etsu Chemical Co., Ltd.) (amine equivalent 430) , stirred at 45°C for 1 hour under nitrogen flow, and then added 3,3'-bis(1-hydroxyl-1-trifluoromethyl-2,2,2-trifluoroethyl)-4,4' - 6.1 parts by mass of methylene diphenylamine (hereinafter, referred to as HFA-MDA) was stirred at 45° C. for 2 hours to react. Next, the temperature of the reaction solution was raised, and water of condensation was azeotropically removed with toluene under a nitrogen stream while maintaining the temperature at about 160°C. When it was confirmed that a predetermined amount of w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com