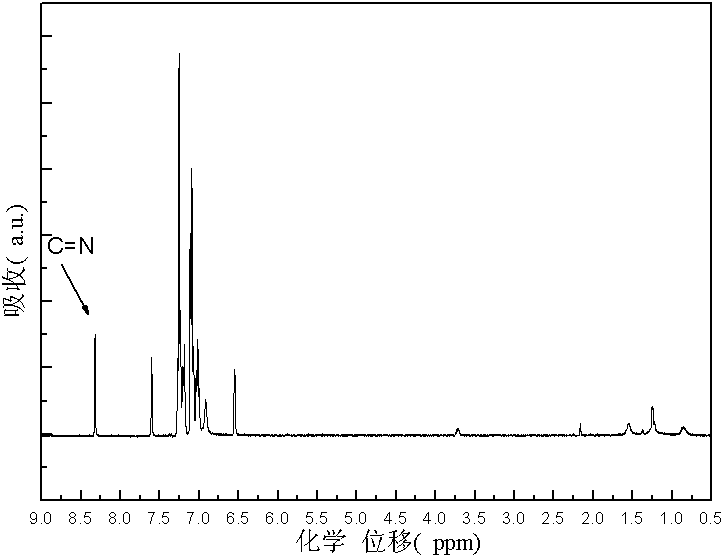
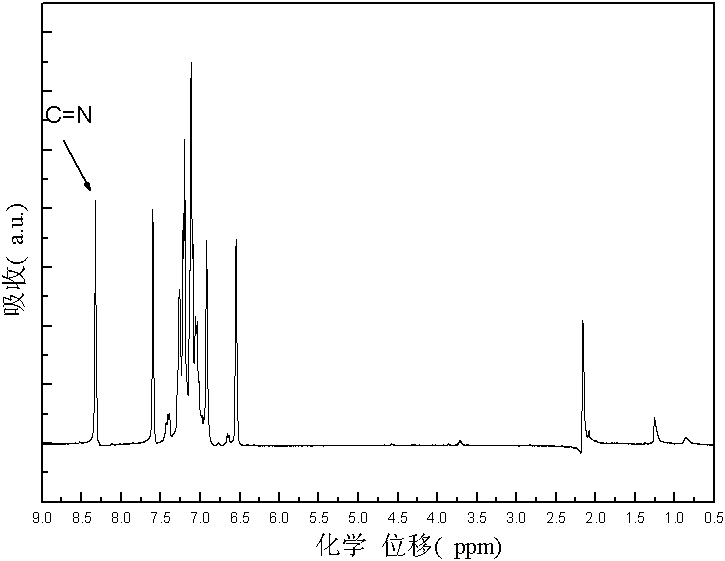
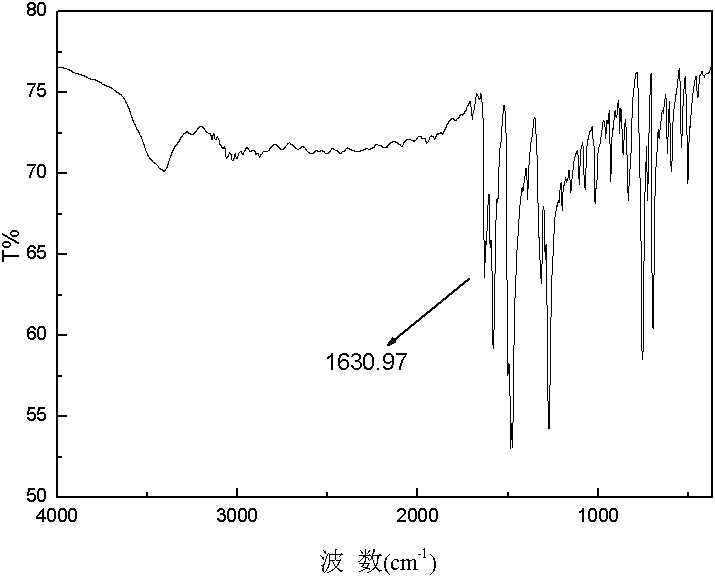
Furaldehyde-triphenylamine Schiff base and furaldehyde-triphenylamine poly Schiff base as well as preparation methods thereof
A technology of triphenylamine poly-Schiff base and triphenylamine-Schiff base, which is applied in the field of Schiff base and poly-Schiff base and its preparation, and can solve problems such as low adhesion, difficult coating processing, and difficult poly-Schiff base polymers, etc. problem, to achieve good adhesion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0028] Specific embodiment one: the present invention adopts the method for electrochemical polymerization, and specific implementation steps are as follows:
[0029] 1. Synthesis of monomers: Dissolve monoaminotriphenylamine in an organic solvent, then add furyl formaldehyde, and then add catalyst glacial acetic acid; then heat and stir under nitrogen protection to 110°C~112°C, and at 110°C~112°C Condensation and reflux reaction at ℃ for 3-5 hours to obtain a transparent orange-yellow solution. Distill the obtained transparent orange-yellow solution under reduced pressure to obtain a solid. Recrystallize the obtained solid for 1-3 times and dry it in vacuum to obtain N-furan Methylene-N'-diphenyl-N'-4-aniline solid; the molar ratio of the monoaminotriphenylamine and furan formaldehyde is 1: (1-3); the organic solvent is toluene, dimethyl Formamide, tetrahydrofuran, dimethylacetamide or N-methylpyrrolidone; the molecular formula of the N-furyl methylene-N'-diphenyl-N'-4-anilin...
specific Embodiment approach 2
[0035] Specific embodiment two: the difference between this embodiment and specific embodiment one is: the molar ratio in the step one is: monoaminotriphenylamine: furfural = 1: 1.5. Others are the same as in the first embodiment.
specific Embodiment approach 3
[0036] Embodiment 3: This embodiment has the same point as Embodiment 1 or Embodiment 2: in step 1, the reaction time of condensation and reflux at 110° C. to 112° C. is 4 hours. Others are the same as those in the first or second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com