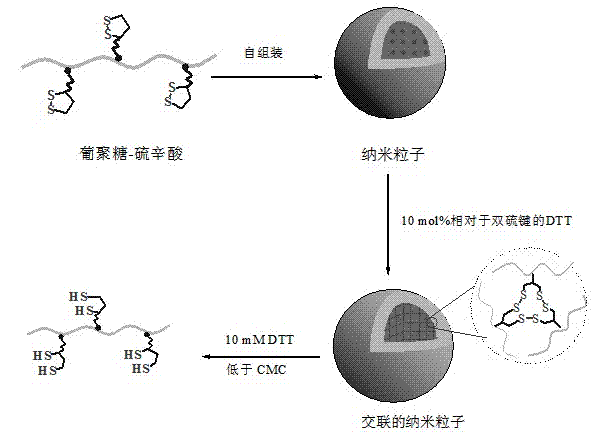
Thioctic acid-modified hydrophilic polymer for side chain
A technology of hydrophilic polymer and amphiphilic polymer, applied in the field of modified hydrophilic polymer, can solve problems such as environmentally sensitive nano-drug carriers, improve the encapsulation efficiency, improve the stability, and overcome the easy leakage Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
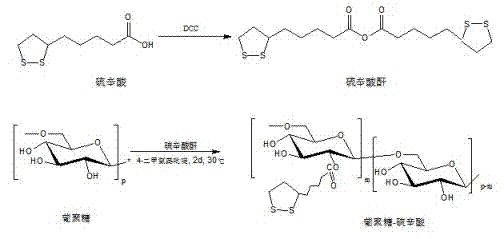
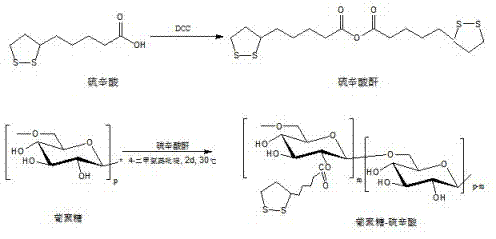
[0039] Embodiment one, synthetic polymer Dex-LA ( M ndextran =20 kDa, DS = 80%)
[0040] Under the protection of argon, dissolve lipoic acid (0.639 g, 3.10 mmol) in 10 mL of dichloromethane, add it into a 50 mL Schlenk vacuum-sealed bottle, and dissolve DCC in 5.0 mL of dichloromethane under argon (0.384g, 1.86 mmol) into a sealed bottle, put the bottle in an oil bath at 30°C, stir and react for 22 hours, cool, filter to remove the urea generated in the reaction, spin the filtrate to obtain lipoic anhydride after removing the solvent .
[0041] The lipoic anhydride obtained above was added to 3 mL of anhydrous-treated dimethyl sulfoxide. Add dextran (0.25 g, 1.55 mmol AHG) dissolved in 19 mL of dimethyl sulfoxide to a 50 mL three-neck flask. Under argon protection, add lipoic anhydride and 2 mL of dimethyl sulfoxide 4-Dimethylaminopyridine (0.189g, 1.55 mmol), the reactor was placed in an oil bath at 30°C, and after stirring for 48 hours, it was precipitated into cold eth...
Embodiment 2
[0042] Embodiment two, synthetic polymer Dex-LA ( M ndextran =20 kDa, DS = 20%)
[0043] Under the protection of argon, dissolve lipoic acid (0.255 g, 1.24 mmol) in 10 mL of dichloromethane, add it to a 50 mL Schlenk vacuum-sealed bottle, and dissolve DCC in 5.0 mL of dichloromethane under argon Add (0.154g, 0.74 mmol) into a sealed bottle, put the bottle in an oil bath at 30°C, stir and react for 22 hours, cool, filter to remove the urea generated in the reaction, and spin the filtrate to obtain lipoic anhydride after removing the solvent .
[0044] The lipoic anhydride obtained above was added to 3 mL of anhydrous-treated dimethyl sulfoxide. Add Dextran (0.25 g, 1.55 mmol AHG) dissolved in 19 mL of dimethyl sulfoxide to a 50 mL three-necked flask, and then add lipoic anhydride and 4-dimethylamino dissolved in 2 mL of dimethyl sulfoxide in sequence under argon protection Pyridine (0.076g, 0.62 mmol), the reactor was placed in an oil bath at 30°C, and after stirring for 4...
Embodiment 3
[0045] Embodiment three, synthetic polymer Dex-LA ( M ndextran =70 kDa, DS = 40%)
[0046] Under the protection of argon, dissolve lipoic acid (0.352 g, 1.71 mmol) in 10 mL of dichloromethane, add it into a 50 mL Schlenk vacuum-sealed bottle, and dissolve DCC in 5.0 mL of dichloromethane under argon Add (0.212g, 1.03 mmol) into a sealed bottle, put the bottle in an oil bath at 30°C, stir and react for 22 hours, cool, filter to remove the urea generated in the reaction, spin the filtrate to obtain lipoic anhydride after removing the solvent .
[0047] The lipoic anhydride obtained above was added to 3 mL of anhydrous-treated dimethyl sulfoxide. Add Dextran (0.25 g, 1.55 mmol AHG) dissolved in 19 mL of dimethyl sulfoxide to a 50 mL three-necked flask, and then add lipoic anhydride and 4-dimethylamino dissolved in 2 mL of dimethyl sulfoxide in sequence under argon protection Pyridine (0.104g, 0.86 mmol), the reactor was placed in an oil bath at 30°C, and after stirring for 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com