Oral disintegrating tablets of microcrystallites of aripiprazole composition and preparation method thereof
A technology of orally disintegrating tablets and aripiprazole, which is applied in the field of orally disintegrating tablets containing aripiprazole composition microcrystals and its preparation, can solve the problems that cannot reflect the advantages of fast dissolution of orally disintegrating tablets, Achieve the effect of good taste, convenient taking, and improved taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
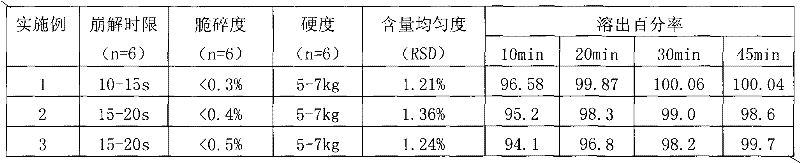
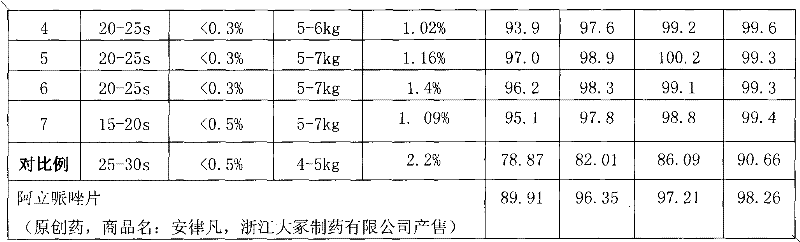
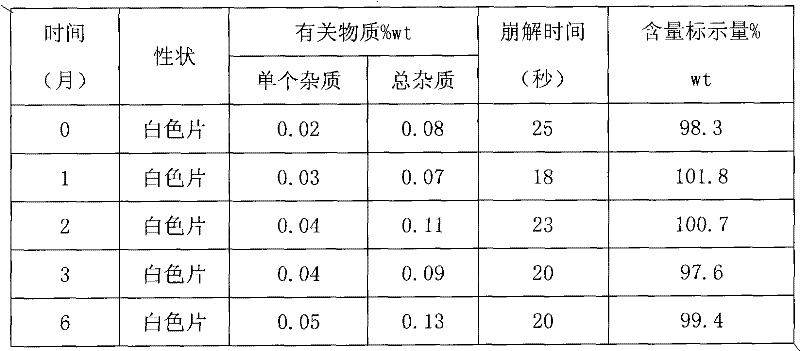
Examples
Embodiment 1
[0052] Example 1. The orally disintegrating tablet of microcrystalline aripiprazole composition consists of:
[0053] Aripiprazole composition microcrystalline (2.5 parts of aripiprazole + 5 parts of lactose) 7.5 parts
[0054] 15 parts lactose (filler)
[0055] Mannitol 59.4 parts (filler)
[0056] Optimized microcrystalline cellulose 10 parts (filler)
[0057]6 parts of crospovidone (disintegrant)
[0058] Sweet orange essence 0.5 parts (flavoring agent)
[0059] 0.6 parts of aspartame (flavoring agent)
[0060] 1.0 parts of magnesium stearate (lubricant);
[0061] Preparation:
[0062] Mix the microcrystalline aripiprazole composition with lactose passed through a 40-mesh sieve to prepare mixture A; mannitol, optimized microcrystalline cellulose, crospovidone, sweet orange essence, and aspartame were passed through a 40-mesh sieve respectively After sieving, mix uniformly to obtain mixture B, mix mixture A and mixture B uniformly in equal increments, add magnesium st...
Embodiment 2
[0063] Example 2. The orally disintegrating tablet of microcrystalline aripiprazole composition consists of:
[0064] Microcrystalline Aripiprazole Composition
[0065] (5 parts of aripiprazole + 5 parts of lactose) 10 parts
[0066] Mannitol 72.9 parts (filler)
[0067] 5 parts microcrystalline cellulose (filler)
[0068] Low-substituted hydroxypropyl cellulose 6 parts (disintegrant)
[0069] 3 parts of croscarmellose sodium (disintegrant)
[0070] Micropowder silica gel 0.5 part (lubricant)
[0071] Strawberry essence 0.5 parts (flavoring agent)
[0072] 0.6 parts of aspartame (flavoring agent)
[0073] 1.5 parts of magnesium stearate (lubricant);
[0074] Preparation:
[0075] Mix the microcrystalline aripiprazole composition with mannitol passing through a 40-mesh sieve to prepare mixture A; microcrystalline cellulose, low-substituted hydroxypropyl cellulose, croscarmellose sodium, micropowder silica gel, strawberry essence and aspartame were respectively passed t...
Embodiment 3
[0076] Example 3. Aripiprazole composition The composition of microcrystalline orally disintegrating tablets by mass is:
[0077] Microcrystalline Aripiprazole Composition
[0078] (2 parts of aripiprazole + 2 parts of lactose) 4 parts
[0079] Mannitol 75 parts (filler)
[0080] Optimized microcrystalline cellulose 10 parts (filler)
[0081] 8 parts of crospovidone (disintegrant)
[0082] Sweet orange essence 1.5 parts (flavoring agent)
[0083] Aspartame 0.5 parts (flavoring agent)
[0084] Micropowder silica gel 0.5 part (lubricant)
[0085] 0.5 parts of magnesium stearate (lubricant);
[0086] Preparation:
[0087] Mix the microcrystalline aripiprazole composition with mannitol passing through a 40-mesh sieve to prepare mixture A; optimize microcrystalline cellulose, crospovidone, sweet orange essence, and aspartame after passing through a 40-mesh sieve Mix uniformly to prepare mixture B, mix mixture A and mixture B uniformly by adding equal amounts, add micropowde...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com