Micromolecule selective depressant for STAT3 (Signal transducer and activator of transcription 3), as well as preparation method and applications thereof
A technology of molecular selectivity and inhibitors, applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, organic active ingredients, etc., can solve the problems of difficult medicinal use and poor bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
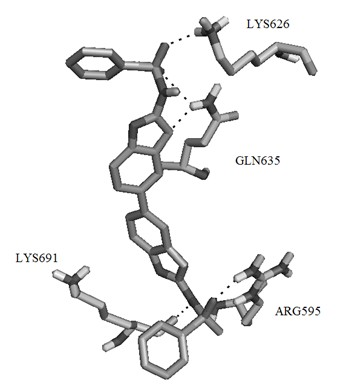
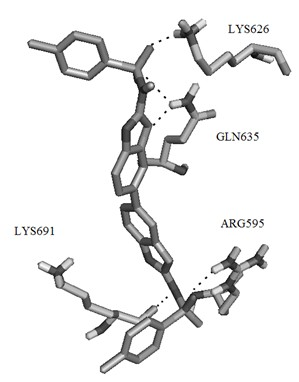
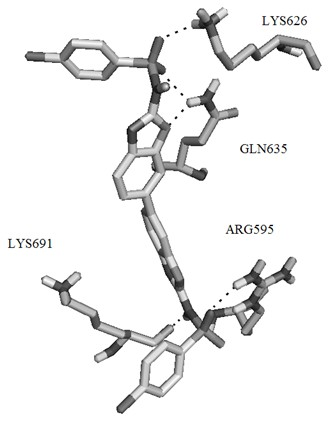
[0057] Example 1 Virtual screening of small molecule selective inhibitors based on STAT3 protein structure.
[0058] The three-dimensional structure of STAT3β homodimer has been used in the screening of STAT3 small molecule selective inhibitors since it was determined in 1998. Its three-dimensional structure shows that STAT3β dimer occurs in the SH2 region of two STAT3 monomers. The two SH2 regions are connected together by their respective monomer fragments, and phosphoryl tyrosine Y705 is located in this region, which is an amino acid residue with important biological functions in STAT3β. Four adjacent amino acid residues are connected to The cavities on the SH2 domain of another monomer bind together.
[0059] In order to obtain organic small molecules that can inhibit or hinder the formation of STAT3β dimer, the protein crystal coded as 1BG1 was obtained from Protein Data Bank. The databases used in the virtual screening include Specs, Maybridge, these two databases inclu...
Embodiment 2
[0061] Example 2 Synthesis of N-bis(methylmercapto)methylene benzenesulfonamides.
[0062] 1 mmol R 1 Dissolve the base-substituted benzenesulfonamide in 2ml of DMF, add 80mg of NaOH, stir at room temperature to form a suspension, cool in an ice-water bath to 0°C, add 1mmol of carbon disulfide dropwise, complete the addition in about 3min, stir for 30min, and add 2mmol of dimethyl sulfate dropwise , about 30min after adding, react at room temperature for 2h, pour the reaction solution into a sufficient amount of water, precipitate a white solid, filter, wash with water, and dry to obtain the product N-bis(methylmercapto)methylene substituted benzenesulfonamide compound 2a~2l, Characterize the product. The reaction formula for this reaction is:
[0063] .
[0064] R 1 The base is different, the product is different, and the data of the characterization is also different. The detailed results Figure 7 .
Embodiment 3
[0065] Example 3 Synthesis of 2,2'-bis(benzenesulfonylamino)-6,6-bibenzoxazole.
[0066] The synthetic reaction formula of 2,2'-bis(benzenesulfonylamino)-6,6-bibenzoxazole is:
[0067] .
[0068] According to the above reaction formula, take 1mmol 3,3'-dihydroxybenzidinediamine and dissolve it in 8ml DMF, add 0.2ml of 5M sodium hydroxide solution, stir at room temperature for 30min, add dropwise 2mmol N-bis(methylmercapto)methylene The solution of benzenesulfonamide (2a) in 2ml of DMF was heated up to 150°C for 8h after the dropwise completion. After the reaction is complete, cool down to room temperature, add 2ml of acetic acid, pour into a sufficient amount of water, precipitate a solid, filter, wash with water, and dry to obtain the product 2,2'-bis(benzenesulfonylamino)-6,6-bibenzoxazole (DABB-1), yield 75%. Characterize the product, the result: 1 H NMR (300 MHz, DMSO): δ7.94 (m, 4H), 7.87 (s, 1H), 7.60 (m, 8H), 7.37 (d, J = 7.0 Hz, 2H); 13 C NMR (75 MHz, DMSO): δ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com